The Head-Direction Signal Plays a Functional Role as a Neural Compass during Navigation
- PMID: 28416119
- PMCID: PMC5425164
- DOI: 10.1016/j.cub.2017.03.033
The Head-Direction Signal Plays a Functional Role as a Neural Compass during Navigation
Erratum in
-
The Head-Direction Signal Plays a Functional Role as a Neural Compass during Navigation.Curr Biol. 2017 Aug 7;27(15):2406. doi: 10.1016/j.cub.2017.07.032. Curr Biol. 2017. PMID: 28787596 No abstract available.
Abstract
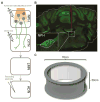
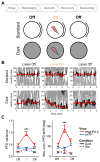
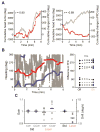
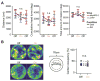
The rat limbic system contains head direction (HD) cells that fire according to heading in the horizontal plane, and these cells are thought to provide animals with an internal compass. Previous work has found that HD cell tuning correlates with behavior on navigational tasks, but a direct, causal link between HD cells and navigation has not been demonstrated. Here, we show that pathway-specific optogenetic inhibition of the nucleus prepositus caused HD cells to become directionally unstable under dark conditions without affecting the animals' locomotion. Then, using the same technique, we found that this decoupling of the HD signal in the absence of visual cues caused the animals to make directional homing errors and that the magnitude and direction of these errors were in a range that corresponded to the degree of instability observed in the HD signal. These results provide evidence that the HD signal plays a causal role as a neural compass in navigation.
Keywords: brainstem; head direction cell; navigation; optogenetics; path integration; thalamus.
Copyright © 2017 Elsevier Ltd. All rights reserved.
Conflict of interest statement
The authors declare no competing financial interests.
Figures


References
-
- Gallistel CR. The Organization of Learning. Cambridge: MIT Press; 1990.
-
- Etienne AS, Jeffery KJ. Path integration in mammals. Hippocampus. 2004;14:180–192. - PubMed
-
- Moser EI, Kropff E, Moser MB. Place cells, grid cells, and the brain’s spatial representation system. Ann Rev Neurosci. 2008;31:69–89. - PubMed
-
- O’Keefe J, Nadel L. The Hippocampus as a Cognitive Map. Oxford: Oxford University Press; 1978.
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

