Foxp3 Reprograms T Cell Metabolism to Function in Low-Glucose, High-Lactate Environments
- PMID: 28416194
- PMCID: PMC5462872
- DOI: 10.1016/j.cmet.2016.12.018
Foxp3 Reprograms T Cell Metabolism to Function in Low-Glucose, High-Lactate Environments
Abstract
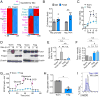
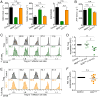
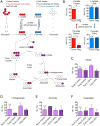
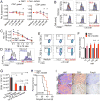
Immune cells function in diverse metabolic environments. Tissues with low glucose and high lactate concentrations, such as the intestinal tract or ischemic tissues, frequently require immune responses to be more pro-tolerant, avoiding unwanted reactions against self-antigens or commensal bacteria. T-regulatory cells (Tregs) maintain peripheral tolerance, but how Tregs function in low-glucose, lactate-rich environments is unknown. We report that the Treg transcription factor Foxp3 reprograms T cell metabolism by suppressing Myc and glycolysis, enhancing oxidative phosphorylation, and increasing nicotinamide adenine dinucleotide oxidation. These adaptations allow Tregs a metabolic advantage in low-glucose, lactate-rich environments; they resist lactate-mediated suppression of T cell function and proliferation. This metabolic phenotype may explain how Tregs promote peripheral immune tolerance during tissue injury but also how cancer cells evade immune destruction in the tumor microenvironment. Understanding Treg metabolism may therefore lead to novel approaches for selective immune modulation in cancer and autoimmune diseases.
Keywords: T cell metabolism; immune regulation; immunometabolism.
Copyright © 2017 Elsevier Inc. All rights reserved.
Figures



Comment in
-
Treg Cells Survive and Thrive in Inhospitable Environments.Cell Metab. 2017 Jun 6;25(6):1213-1215. doi: 10.1016/j.cmet.2017.05.012. Cell Metab. 2017. PMID: 28591629
References
MeSH terms
Substances
Grants and funding
- K23 DK101600/DK/NIDDK NIH HHS/United States
- R01 DK098656/DK/NIDDK NIH HHS/United States
- R01 MH108592/MH/NIMH NIH HHS/United States
- R01 DK106243/DK/NIDDK NIH HHS/United States
- K08 DK092282/DK/NIDDK NIH HHS/United States
- P01 AI073489/AI/NIAID NIH HHS/United States
- K08 AI095353/AI/NIAID NIH HHS/United States
- R56 AI095276/AI/NIAID NIH HHS/United States
- P30 CA016520/CA/NCI NIH HHS/United States
- R01 OD010944/OD/NIH HHS/United States
- R01 AI073938/AI/NIAID NIH HHS/United States
- P30 DK019525/DK/NIDDK NIH HHS/United States
- R01 NS021328/NS/NINDS NIH HHS/United States
- R33 CA182384/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

