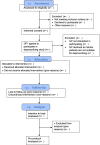
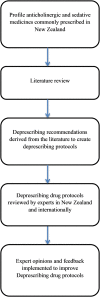
Deprescribing anticholinergic and sedative medicines: protocol for a Feasibility Trial (DEFEAT-polypharmacy) in residential aged care facilities
- PMID: 28416498
- PMCID: PMC5775460
- DOI: 10.1136/bmjopen-2016-013800
Deprescribing anticholinergic and sedative medicines: protocol for a Feasibility Trial (DEFEAT-polypharmacy) in residential aged care facilities
Abstract
Introduction: Targeted deprescribing of anticholinergic and sedative medicines can lead to positive health outcomes in older people; as they have been associated with cognitive and physical functioning decline. This study will examine whether the proposed intervention is feasible at reducing the prescription of anticholinergic and sedative medicines in older people.
Methods and analysis: The Standard Protocol Items: Recommendations for Interventional trials (SPIRIT checklist) was used to develop and report the protocol. Single group (precomparison and postcomparison) feasibility study design.
Study population: 3 residential care homes have been recruited.
Intervention: This will involve a New Zealand registered pharmacist using peer-reviewed deprescribing guidelines, to recommend to general practitioners (GPs), sedative and anticholinergic medicines that can be deprescribed. The cumulative use of anticholinergic and sedative medicines for each participant will be quantified, using the Drug Burden Index (DBI).
Outcomes: The primary outcome will be the change in the participants' DBI total and DBI PRN 3 and 6 months after implementing the deprescribing intervention. Secondary outcomes will include the number of recommendations taken up by the GP, participants' cognitive functioning, depression, quality of life, activities of daily living and number of falls.
Data collection points: Participants' demographic and clinical data will be collected at the time of enrolment, along with the DBI. Outcome measures will be collected at the time of enrolment, 3 and 6 months' postenrolment.
Ethics and dissemination: Ethics approval has been granted by the Human Disability and Ethics Committee. Ethical approval number (16/NTA/61).
Trial registration number: Pre-results; ACTRN12616000721404.
Keywords: Deprescribing; Elderly; Feasibility study; anticholinergics; drug burden index; sedatives.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://www.bmj.com/company/products-services/rights-and-licensing/.
Conflict of interest statement
Competing interestsNone declared.
Figures
Similar articles
-
DEFEAT-polypharmacy: deprescribing anticholinergic and sedative medicines feasibility trial in residential aged care facilities.Int J Clin Pharm. 2019 Feb;41(1):167-178. doi: 10.1007/s11096-019-00784-9. Epub 2019 Jan 18. Int J Clin Pharm. 2019. PMID: 30659492 Clinical Trial.
-
Deprescribing to reduce polypharmacy: study protocol for a randomised controlled trial assessing deprescribing of anticholinergic and sedative drugs in a cohort of frail older people living in the community.Trials. 2021 Nov 3;22(1):766. doi: 10.1186/s13063-021-05711-w. Trials. 2021. PMID: 34732234 Free PMC article.
-
Decreasing the load? Is a Multidisciplinary Multistep Medication Review in older people an effective intervention to reduce a patient's Drug Burden Index? Protocol of a randomised controlled trial.BMJ Open. 2015 Dec 23;5(12):e009213. doi: 10.1136/bmjopen-2015-009213. BMJ Open. 2015. PMID: 26700279 Free PMC article. Clinical Trial.
-
Drug Burden Index in older adults: theoretical and practical issues.Clin Interv Aging. 2014 Sep 9;9:1503-15. doi: 10.2147/CIA.S66660. eCollection 2014. Clin Interv Aging. 2014. PMID: 25246778 Free PMC article. Review.
-
Anticholinergic and sedative medicines - prescribing considerations for people with dementia.Aust Fam Physician. 2012 Jan-Feb;41(1-2):45-9. Aust Fam Physician. 2012. PMID: 22276284 Review.
Cited by
-
DEFEAT-polypharmacy: deprescribing anticholinergic and sedative medicines feasibility trial in residential aged care facilities.Int J Clin Pharm. 2019 Feb;41(1):167-178. doi: 10.1007/s11096-019-00784-9. Epub 2019 Jan 18. Int J Clin Pharm. 2019. PMID: 30659492 Clinical Trial.
-
Anticholinergic deprescribing interventions for reducing risk of cognitive decline or dementia in older adults with and without prior cognitive impairment.Cochrane Database Syst Rev. 2023 Dec 8;12(12):CD015405. doi: 10.1002/14651858.CD015405.pub2. Cochrane Database Syst Rev. 2023. PMID: 38063254 Free PMC article.
-
The Use of Medicines with Anti-cholinergic Properties and Their Health Impacts among Hospitalised Malaysian Geriatric Patients.Malays J Med Sci. 2019 Mar;26(2):77-87. doi: 10.21315/mjms2019.26.2.9. Epub 2019 Apr 30. Malays J Med Sci. 2019. PMID: 31447611 Free PMC article.
-
The past, present and future of anticholinergic drugs.Ther Adv Psychopharmacol. 2023 Sep 8;13:20451253231176375. doi: 10.1177/20451253231176375. eCollection 2023. Ther Adv Psychopharmacol. 2023. PMID: 37701889 Free PMC article. Review.
-
Exposure to anticholinergic and sedative medication is associated with impaired functioning in older people with vertigo, dizziness and balance disorders-Results from the longitudinal multicenter study MobilE-TRA.Front Pharmacol. 2023 Mar 3;14:1136757. doi: 10.3389/fphar.2023.1136757. eCollection 2023. Front Pharmacol. 2023. PMID: 36937862 Free PMC article.
References
-
- Garfinkel D, Zur-Gil S, Ben-Israel J. The war against polypharmacy: a new cost-effective geriatric-palliative approach for improving drug therapy in disabled elderly people. Isr Med Assoc J 2007;9:430–4. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources