Erythrocyte sialoglycoproteins engage Siglec-9 on neutrophils to suppress activation
- PMID: 28416510
- PMCID: PMC5465837
- DOI: 10.1182/blood-2016-11-751636
Erythrocyte sialoglycoproteins engage Siglec-9 on neutrophils to suppress activation
Abstract
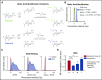
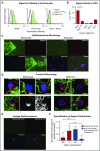
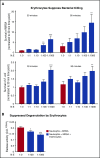
Healthy blood neutrophils are functionally quiescent in the bloodstream, have a short lifespan, and exit the circulation to carry out innate immune functions, or undergo rapid apoptosis and macrophage-mediated clearance to mitigate host tissue damage. Limitation of unnecessary intravascular neutrophil activation is also important to prevent serious inflammatory pathologies. Because neutrophils become easily activated after purification, we carried out ex vivo comparisons with neutrophils maintained in whole blood. We found a difference in activation state, with purified neutrophils showing signs of increased reactivity: shedding of l-selectin, CD11b upregulation, increased oxidative burst, and faster progression to apoptosis. We discovered that erythrocytes suppressed neutrophil activation ex vivo and in vitro, including reduced l-selectin shedding, oxidative burst, chemotaxis, neutrophil extracellular trap formation, bacterial killing, and induction of apoptosis. Selective and specific modification of sialic acid side chains on erythrocyte surfaces with mild sodium metaperiodate oxidation followed by aldehyde quenching with 4-methyl-3-thiosemicarbazide reduced neutrophil binding to erythrocytes and restored neutrophil activation. By enzyme-linked immunosorbent assay and immunofluorescence, we found that glycophorin A, the most abundant sialoglycoprotein on erythrocytes, engaged neutrophil Siglec-9, a sialic acid-recognizing receptor known to dampen innate immune cell activation. These studies demonstrate a previously unsuspected role for erythrocytes in suppressing neutrophils ex vivo and in vitro and help explain why neutrophils become easily activated after separation from whole blood. We propose that a sialic acid-based "self-associated molecular pattern" on erythrocytes also helps maintain neutrophil quiescence in the bloodstream. Our findings may be relevant to some prior experimental and clinical studies of neutrophils.
© 2017 by The American Society of Hematology.
Figures


Similar articles
-
Decreased erythrocyte binding of Siglec-9 increases neutrophil activation in sickle cell disease.Blood Cells Mol Dis. 2020 Mar;81:102399. doi: 10.1016/j.bcmd.2019.102399. Epub 2019 Dec 20. Blood Cells Mol Dis. 2020. PMID: 31901888 Free PMC article.
-
Host and pathogen hyaluronan signal through human siglec-9 to suppress neutrophil activation.J Mol Med (Berl). 2016 Feb;94(2):219-33. doi: 10.1007/s00109-015-1341-8. Epub 2015 Sep 28. J Mol Med (Berl). 2016. PMID: 26411873 Free PMC article.
-
Molecular mimicry of host sialylated glycans allows a bacterial pathogen to engage neutrophil Siglec-9 and dampen the innate immune response.Blood. 2009 Apr 2;113(14):3333-6. doi: 10.1182/blood-2008-11-187302. Epub 2009 Feb 4. Blood. 2009. PMID: 19196661 Free PMC article.
-
Targeting Neutrophils in Severe Asthma via Siglec-9.Int Arch Allergy Immunol. 2018;175(1-2):5-15. doi: 10.1159/000484873. Epub 2018 Jan 6. Int Arch Allergy Immunol. 2018. PMID: 29306942 Review.
-
Regulation of neutrophil functions through inhibitory receptors: an emerging paradigm in health and disease.Immunol Rev. 2016 Sep;273(1):140-55. doi: 10.1111/imr.12457. Immunol Rev. 2016. PMID: 27558333 Review.
Cited by
-
Sialoglycan recognition is a common connection linking acidosis, zinc, and HMGB1 in sepsis.Proc Natl Acad Sci U S A. 2021 Mar 9;118(10):e2018090118. doi: 10.1073/pnas.2018090118. Proc Natl Acad Sci U S A. 2021. PMID: 33658363 Free PMC article.
-
Aberrant GPA expression and regulatory function of red blood cells in sickle cell disease.Blood Adv. 2024 Apr 9;8(7):1687-1697. doi: 10.1182/bloodadvances.2023011611. Blood Adv. 2024. PMID: 38231087 Free PMC article.
-
Effect of Vitamin D3 in combination with Omega-3 Polyunsaturated Fatty Acids on NETosis in Type 2 Diabetes Mellitus Patients.Oxid Med Cell Longev. 2021 Oct 22;2021:8089696. doi: 10.1155/2021/8089696. eCollection 2021. Oxid Med Cell Longev. 2021. PMID: 34721760 Free PMC article.
-
Dual actions of group B Streptococcus capsular sialic acid provide resistance to platelet-mediated antimicrobial killing.Proc Natl Acad Sci U S A. 2019 Apr 9;116(15):7465-7470. doi: 10.1073/pnas.1815572116. Epub 2019 Mar 25. Proc Natl Acad Sci U S A. 2019. PMID: 30910970 Free PMC article.
-
Tumor cell-intrinsic and tumor microenvironmental conditions co-determine signaling by the glycoimmune checkpoint receptor Siglec-7.Cell Mol Life Sci. 2023 May 30;80(6):169. doi: 10.1007/s00018-023-04816-6. Cell Mol Life Sci. 2023. PMID: 37253806 Free PMC article.
References
-
- Mohandas N. Structure and composition of the erythrocyte. In: Kaushansky EA, ed. Williams Hematology. New York, NY: McGraw-Hill; 2016:461-478.
-
- Silvestre-Roig C, Hidalgo A, Soehnlein O. Neutrophil heterogeneity: implications for homeostasis and pathogenesis. Blood. 2016;127(18):2173-2181. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

