Semaphorin 4D inhibits neutrophil activation and is involved in the pathogenesis of neutrophil-mediated autoimmune vasculitis
- PMID: 28416516
- PMCID: PMC5738596
- DOI: 10.1136/annrheumdis-2016-210706
Semaphorin 4D inhibits neutrophil activation and is involved in the pathogenesis of neutrophil-mediated autoimmune vasculitis
Abstract
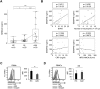
Objectives: Inappropriate activation of neutrophils plays a pathological role in antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV). The aim of this study was to investigate the functions of semaphorin 4D (SEMA4D) in regulation of neutrophil activation, and its involvement in AAV pathogenesis.
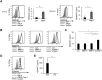
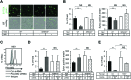
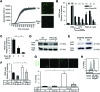
Methods: Serum levels of soluble SEMA4D were evaluated by ELISA. Blood cell-surface expression of membrane SEMA4D was evaluated by flow cytometry. To determine the functional interactions between neutrophil membrane SEMA4D and endothelial plexin B2, wild-type and SEMA4D-/- mice neutrophils were cultured with an endothelial cell line (MS1) stained with SYTOX green, and subjected to neutrophil extracellular trap (NET) formation assays. The efficacy of treating human neutrophils with recombinant plexin B2 was assessed by measuring the kinetic oxidative burst and NET formation assays.
Results: Serum levels of soluble SEMA4D were elevated in patients with AAV and correlated with disease activity scores. Cell-surface expression of SEMA4D was downregulated in neutrophils from patients with AAV, a consequence of proteolytic cleavage of membrane SEMA4D. Soluble SEMA4D exerted pro-inflammatory effects on endothelial cells. Membranous SEMA4D on neutrophils bound to plexin B2 on endothelial cells, and this interaction decreased NET formation. Recombinant plexin B2 suppressed neutrophil Rac1 activation through SEMA4D's intracellular domain, and inhibited pathogen-induced or ANCA-induced oxidative burst and NET formation.
Conclusions: Neutrophil surface SEMA4D functions as a negative regulator of neutrophil activation. Proteolytic cleavage of SEMA4D as observed in patients with AAV may amplify neutrophil-mediated inflammatory responses. SEMA4D is a promising biomarker and potential therapeutic target for AAV.
Keywords: Autoimmune Diseases; Granulomatosis with polyangiitis; Inflammation; Systemic vasculitis.
© Article author(s) (or their employer(s) unless otherwise stated in the text of the article) 2017. All rights reserved. No commercial use is permitted unless otherwise expressly granted.
Conflict of interest statement
Competing interests: None declared.
Figures
Similar articles
-
Transcription factor Nrf2 activation regulates NETosis, endothelial injury, and kidney disease in myeloperoxidase-positive antineutrophil cytoplasmic antibody-associated vasculitis.Kidney Int. 2024 Jun;105(6):1291-1305. doi: 10.1016/j.kint.2024.03.010. Epub 2024 Mar 25. Kidney Int. 2024. PMID: 38537677
-
Neutrophil-Derived Semaphorin 4D Induces Inflammatory Cytokine Production of Endothelial Cells via Different Plexin Receptors in Kawasaki Disease.Biomed Res Int. 2020 Dec 16;2020:6663291. doi: 10.1155/2020/6663291. eCollection 2020. Biomed Res Int. 2020. PMID: 33381571 Free PMC article.
-
Protective effect of astaxanthin on ANCA-associated vasculitis.Int Immunopharmacol. 2024 May 10;132:111928. doi: 10.1016/j.intimp.2024.111928. Epub 2024 Mar 26. Int Immunopharmacol. 2024. PMID: 38537540
-
The role of neutrophils in ANCA-associated vasculitis.Immunol Lett. 2024 Dec;270:106933. doi: 10.1016/j.imlet.2024.106933. Epub 2024 Oct 1. Immunol Lett. 2024. PMID: 39362307 Review.
-
At the Bedside: Neutrophil extracellular traps (NETs) as targets for biomarkers and therapies in autoimmune diseases.J Leukoc Biol. 2016 Feb;99(2):265-78. doi: 10.1189/jlb.5BT0615-234R. Epub 2015 Dec 11. J Leukoc Biol. 2016. PMID: 26658004 Free PMC article. Review.
Cited by
-
Semaphorin 4D Induces an Imbalance of Th17/Treg Cells by Activating the Aryl Hydrocarbon Receptor in Ankylosing Spondylitis.Front Immunol. 2020 Sep 8;11:2151. doi: 10.3389/fimmu.2020.02151. eCollection 2020. Front Immunol. 2020. PMID: 33013906 Free PMC article.
-
The Expression of Semaphorin3E in Vagal Ganglion and Lung Tissue Is Related to Airway Hyperresponsiveness in Murine Asthma Model.J Immunol Res. 2023 Dec 11;2023:6459234. doi: 10.1155/2023/6459234. eCollection 2023. J Immunol Res. 2023. PMID: 38111650 Free PMC article.
-
Neutrophils from ANCA-associated vasculitis patients show an increased capacity to activate the complement system via the alternative pathway after ANCA stimulation.PLoS One. 2019 Jun 19;14(6):e0218272. doi: 10.1371/journal.pone.0218272. eCollection 2019. PLoS One. 2019. PMID: 31216309 Free PMC article.
-
Research Advances in the Immunomodulatory Functions of CD100/SEMA4D and Their Roles in Viral Infectious Diseases.Int J Mol Sci. 2025 May 2;26(9):4341. doi: 10.3390/ijms26094341. Int J Mol Sci. 2025. PMID: 40362578 Free PMC article. Review.
-
The Role of Semaphorins in Metabolic Disorders.Int J Mol Sci. 2020 Aug 6;21(16):5641. doi: 10.3390/ijms21165641. Int J Mol Sci. 2020. PMID: 32781674 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

