Simulating Intestinal Transporter and Enzyme Activity in a Physiologically Based Pharmacokinetic Model for Tenofovir Disoproxil Fumarate
- PMID: 28416547
- PMCID: PMC5487657
- DOI: 10.1128/AAC.00105-17
Simulating Intestinal Transporter and Enzyme Activity in a Physiologically Based Pharmacokinetic Model for Tenofovir Disoproxil Fumarate
Abstract
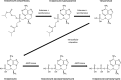
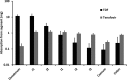
Tenofovir disoproxil fumarate (TDF), a prodrug of tenofovir, has oral bioavailability (25%) limited by intestinal transport (P-glycoprotein), and intestinal degradation (carboxylesterase). However, the influence of luminal pancreatic enzymes is not fully understood. Physiologically based pharmacokinetic (PBPK) modeling has utility for estimating drug exposure from in vitro data. This study aimed to develop a PBPK model that included luminal enzyme activity to inform dose reduction strategies. TDF and tenofovir stability in porcine pancrelipase concentrations was assessed (0, 0.48, 4.8, 48, and 480 U/ml of lipase; 1 mM TDF; 37°C; 0 to 30 min). Samples were analyzed using mass spectrometry. TDF stability and permeation data allowed calculation of absorption rates within a human PBPK model to predict plasma exposure following 6 days of once-daily dosing with 300 mg of TDF. Regional absorption of drug was simulated across gut segments. TDF was degraded by pancrelipase (half-lives of 0.07 and 0.62 h using 480 and 48 U/ml, respectively). Previously reported maximum concentration (Cmax; 335 ng/ml), time to Cmax (Tmax; 2.4 h), area under the concentration-time curve from 0 to 24 h (AUC0-24; 3,045 ng · h/ml), and concentration at 24 h (C24; 48.3 ng/ml) were all within a 0.5-fold difference from the simulated Cmax (238 ng/ml), Tmax (3 h), AUC0-24 (3,036 ng · h/ml), and C24 (42.7 ng/ml). Simulated TDF absorption was higher in duodenum and jejunum than in ileum (p<0.05). These data support that TDF absorption is limited by the action of intestinal lipases. Our results suggest that bioavailability may be improved by protection of drug from intestinal transporters and enzymes, for example, by coadministration of enzyme-inhibiting agents or nanoformulation strategies.
Keywords: HAART; HIV; bioavailability; physiologically based pharmacokinetic modeling; tenofovir disoproxil fumarate.
Copyright © 2017 American Society for Microbiology.
Figures

Similar articles
-
Effect of P-glycoprotein and Cotreatment with Sofosbuvir on the Intestinal Permeation of Tenofovir Disoproxil Fumarate and Tenofovir Alafenamide Fumarate.Pharm Res. 2023 Sep;40(9):2109-2120. doi: 10.1007/s11095-023-03581-2. Epub 2023 Aug 18. Pharm Res. 2023. PMID: 37594591
-
Effect of Pregnancy and Concomitant Antiretrovirals on the Pharmacokinetics of Tenofovir in Women With HIV Receiving Tenofovir Disoproxil Fumarate-Based Antiretroviral Therapy Versus Women With HBV Receiving Tenofovir Disoproxil Fumarate Monotherapy.J Clin Pharmacol. 2021 Mar;61(3):388-393. doi: 10.1002/jcph.1746. Epub 2020 Sep 22. J Clin Pharmacol. 2021. PMID: 32960984 Free PMC article.
-
Comparative Bioavailability of Oral Granule Formulations of the HIV Antiretroviral Drugs Doravirine, Lamivudine, and Tenofovir Disoproxil Fumarate.AAPS PharmSciTech. 2020 Feb 13;21(3):91. doi: 10.1208/s12249-020-1630-6. AAPS PharmSciTech. 2020. PMID: 32060665 Clinical Trial.
-
Pharmacokinetics of oral tenofovir disoproxil fumarate in pregnancy and lactation: a systematic review.Antivir Ther. 2019;24(7):529-540. doi: 10.3851/IMP3341. Antivir Ther. 2019. PMID: 31868655
-
Emtricitabine/tenofovir disoproxil fumarate: a review of its use in HIV-1 pre-exposure prophylaxis.Drugs. 2013 Mar;73(3):279-91. doi: 10.1007/s40265-013-0024-4. Drugs. 2013. PMID: 23444256 Review.
Cited by
-
Pharmacokinetics of tenofovir monoester and association with intracellular tenofovir diphosphate following single-dose tenofovir disoproxil fumarate.J Antimicrob Chemother. 2019 Aug 1;74(8):2352-2359. doi: 10.1093/jac/dkz187. J Antimicrob Chemother. 2019. PMID: 31093649 Free PMC article. Clinical Trial.
-
Increased tenofovir monoester concentrations in patients receiving tenofovir disoproxil fumarate with ledipasvir/sofosbuvir.J Antimicrob Chemother. 2019 Aug 1;74(8):2360-2364. doi: 10.1093/jac/dkz184. J Antimicrob Chemother. 2019. PMID: 31081036 Free PMC article.
References
-
- Pinheiro Edos S, Bruning K, Macedo MF, Siani AC. 2014. Production of antiretroviral drugs in middle- and low-income countries. Antivir Ther 19(Suppl 3):S49–S55. - PubMed
-
- Deeks SG, Barditch-Crovo P, Lietman PS, Hwang F, Cundy KC, Rooney JF, Hellmann NS, Safrin S, Kahn JO. 1998. Safety, pharmacokinetics, and antiretroviral activity of intravenous 9-[2-(R)-(phosphonomethoxy)propyl]adenine, a novel anti-human immunodeficiency virus (HIV) therapy, in HIV-infected adults. Antimicrob Agents Chemother 42:2380–2384. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

