The C-terminal dsRNA-binding domain of Drosophila Dicer-2 is crucial for efficient and high-fidelity production of siRNA and loading of siRNA to Argonaute2
- PMID: 28416567
- PMCID: PMC5473147
- DOI: 10.1261/rna.059915.116
The C-terminal dsRNA-binding domain of Drosophila Dicer-2 is crucial for efficient and high-fidelity production of siRNA and loading of siRNA to Argonaute2
Abstract
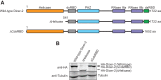
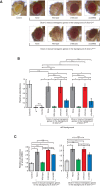
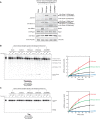
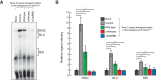
Drosophila Dicer-2 efficiently and precisely produces 21-nucleotide (nt) siRNAs from long double-stranded RNA (dsRNA) substrates and loads these siRNAs onto the effector protein Argonaute2 for RNA silencing. The functional roles of each domain of the multidomain Dicer-2 enzyme in the production and loading of siRNAs are not fully understood. Here we characterized Dicer-2 mutants lacking either the N-terminal helicase domain or the C-terminal dsRNA-binding domain (CdsRBD) (ΔHelicase and ΔCdsRBD, respectively) in vivo and in vitro. We found that ΔCdsRBD Dicer-2 produces siRNAs with lowered efficiency and length fidelity, producing a smaller ratio of 21-nt siRNAs and higher ratios of 20- and 22-nt siRNAs in vivo and in vitro. We also found that ΔCdsRBD Dicer-2 cannot load siRNA duplexes to Argonaute2 in vitro. Consistent with these findings, we found that ΔCdsRBD Dicer-2 causes partial loss of RNA silencing activity in vivo. Thus, Dicer-2 CdsRBD is crucial for the efficiency and length fidelity in siRNA production and for siRNA loading. Together with our previously published findings, we propose that CdsRBD binds the proximal body region of a long dsRNA substrate whose 5'-monophosphate end is anchored by the phosphate-binding pocket in the PAZ domain. CdsRBD aligns the RNA to the RNA cleavage active site in the RNase III domain for efficient and high-fidelity siRNA production. This study reveals multifunctions of Dicer-2 CdsRBD and sheds light on the molecular mechanism by which Dicer-2 produces 21-nt siRNAs with a high efficiency and fidelity for efficient RNA silencing.
Keywords: Dicer; RNA silencing; dsRNA; fidelity; siRNA.
© 2017 Kandasamy et al.; Published by Cold Spring Harbor Laboratory Press for the RNA Society.
Figures



Similar articles
-
Phosphate-binding pocket in Dicer-2 PAZ domain for high-fidelity siRNA production.Proc Natl Acad Sci U S A. 2016 Dec 6;113(49):14031-14036. doi: 10.1073/pnas.1612393113. Epub 2016 Nov 21. Proc Natl Acad Sci U S A. 2016. PMID: 27872309 Free PMC article.
-
Inorganic phosphate blocks binding of pre-miRNA to Dicer-2 via its PAZ domain.EMBO J. 2014 Feb 18;33(4):371-84. doi: 10.1002/embj.201387176. Epub 2014 Jan 31. EMBO J. 2014. PMID: 24488111 Free PMC article.
-
Loquacious-PD facilitates Drosophila Dicer-2 cleavage through interactions with the helicase domain and dsRNA.Proc Natl Acad Sci U S A. 2017 Sep 19;114(38):E7939-E7948. doi: 10.1073/pnas.1707063114. Epub 2017 Sep 5. Proc Natl Acad Sci U S A. 2017. PMID: 28874570 Free PMC article.
-
The RNAi pathway initiated by Dicer-2 in Drosophila.Cold Spring Harb Symp Quant Biol. 2006;71:39-44. doi: 10.1101/sqb.2006.71.008. Cold Spring Harb Symp Quant Biol. 2006. PMID: 17381278 Review.
-
Cryo-EM structures of human DICER dicing a pre-miRNA substrate.FEBS J. 2024 Jul;291(14):3072-3079. doi: 10.1111/febs.17048. Epub 2024 Jan 10. FEBS J. 2024. PMID: 38151772 Review.
Cited by
-
Untangling the roles of RNA helicases in antiviral innate immunity.PLoS Pathog. 2021 Dec 9;17(12):e1010072. doi: 10.1371/journal.ppat.1010072. eCollection 2021 Dec. PLoS Pathog. 2021. PMID: 34882751 Free PMC article. Review.
-
Drosophila Regnase-1 RNase is required for mRNA and miRNA profile remodelling during larva-to-adult metamorphosis.RNA Biol. 2019 Oct;16(10):1386-1400. doi: 10.1080/15476286.2019.1630799. Epub 2019 Jun 23. RNA Biol. 2019. PMID: 31195914 Free PMC article.
-
Recognition of Arboviruses by the Mosquito Immune System.Biomolecules. 2023 Jul 21;13(7):1159. doi: 10.3390/biom13071159. Biomolecules. 2023. PMID: 37509194 Free PMC article. Review.
-
LOTUS domain protein MARF1 binds CCR4-NOT deadenylase complex to post-transcriptionally regulate gene expression in oocytes.Nat Commun. 2018 Oct 2;9(1):4031. doi: 10.1038/s41467-018-06404-w. Nat Commun. 2018. PMID: 30279526 Free PMC article.
-
SfDicer2 RNA Interference Inhibits Molting and Wing Expansion in Sogatella furcifera.Insects. 2022 Jul 27;13(8):677. doi: 10.3390/insects13080677. Insects. 2022. PMID: 36005304 Free PMC article.
References
-
- Bernstein E, Caudy AA, Hammond SM, Hannon GJ. 2001. Role for a bidentate ribonuclease in the initiation step of RNA interference. Nature 409: 363–366. - PubMed
-
- Bronkhorst AW, van Rij RP. 2014. The long and short of antiviral defense: small RNA-based immunity in insects. Curr Opin Virol 7: 19–28. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
