'WNT-er is coming': WNT signalling in chronic lung diseases
- PMID: 28416592
- PMCID: PMC5537530
- DOI: 10.1136/thoraxjnl-2016-209753
'WNT-er is coming': WNT signalling in chronic lung diseases
Abstract
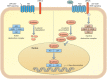
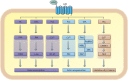
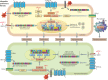
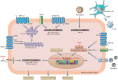
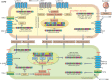
Chronic lung diseases represent a major public health problem with only limited therapeutic options. An important unmet need is to identify compounds and drugs that target key molecular pathways involved in the pathogenesis of chronic lung diseases. Over the last decade, there has been extensive interest in investigating Wingless/integrase-1 (WNT) signalling pathways; and WNT signal alterations have been linked to pulmonary disease pathogenesis and progression. Here, we comprehensively review the cumulative evidence for WNT pathway alterations in chronic lung pathologies, including idiopathic pulmonary fibrosis, pulmonary arterial hypertension, asthma and COPD. While many studies have focused on the canonical WNT/β-catenin signalling pathway, recent reports highlight that non-canonical WNT signalling may also significantly contribute to chronic lung pathologies; these studies will be particularly featured in this review. We further discuss recent advances uncovering the role of WNT signalling early in life, the potential of pharmaceutically modulating WNT signalling pathways and highlight (pre)clinical studies describing promising new therapies for chronic lung diseases.
Keywords: Airway Epithelium; Asthma; Asthma Mechanisms; COPD Pharmacology; COPD ÀÜ Mechanisms; Cytokine Biology; Idiopathic pulmonary fibrosis.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://www.bmj.com/company/products-services/rights-and-licensing/.
Conflict of interest statement
Competing interests: None declared.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
