MicroRNA Profiling Reveals Marker of Motor Neuron Disease in ALS Models
- PMID: 28416596
- PMCID: PMC5452343
- DOI: 10.1523/JNEUROSCI.3582-16.2017
MicroRNA Profiling Reveals Marker of Motor Neuron Disease in ALS Models
Abstract
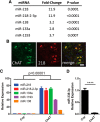
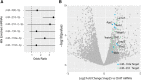
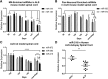
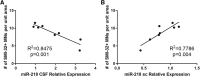
Amyotrophic lateral sclerosis (ALS) is a progressive neurodegenerative disorder marked by the loss of motor neurons (MNs) in the brain and spinal cord, leading to fatally debilitating weakness. Because this disease predominantly affects MNs, we aimed to characterize the distinct expression profile of that cell type to elucidate underlying disease mechanisms and to identify novel targets that inform on MN health during ALS disease time course. microRNAs (miRNAs) are short, noncoding RNAs that can shape the expression profile of a cell and thus often exhibit cell-type-enriched expression. To determine MN-enriched miRNA expression, we used Cre recombinase-dependent miRNA tagging and affinity purification in mice. By defining the in vivo miRNA expression of MNs, all neurons, astrocytes, and microglia, we then focused on MN-enriched miRNAs via a comparative analysis and found that they may functionally distinguish MNs postnatally from other spinal neurons. Characterizing the levels of the MN-enriched miRNAs in CSF harvested from ALS models of MN disease demonstrated that one miRNA (miR-218) tracked with MN loss and was responsive to an ALS therapy in rodent models. Therefore, we have used cellular expression profiling tools to define the distinct miRNA expression of MNs, which is likely to enrich future studies of MN disease. This approach enabled the development of a novel, drug-responsive marker of MN disease in ALS rodents.SIGNIFICANCE STATEMENT Amyotrophic lateral sclerosis (ALS) is a neurodegenerative disease in which motor neurons (MNs) in the brain and spinal cord are selectively lost. To develop tools to aid in our understanding of the distinct expression profiles of MNs and, ultimately, to monitor MN disease progression, we identified small regulatory microRNAs (miRNAs) that were highly enriched or exclusive in MNs. The signal for one of these MN-enriched miRNAs is detectable in spinal tap biofluid from an ALS rat model, where its levels change as disease progresses, suggesting that it may be a clinically useful marker of disease status. Furthermore, rats treated with ALS therapy have restored expression of this MN RNA marker, making it an MN-specific and drug-responsive marker for ALS rodents.
Keywords: ALS; TRAP; miRAP; microRNAs; motor neuron; motor neuron disease.
Copyright © 2017 the authors 0270-6474/17/375574-13$15.00/0.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous
