CD36 receptor regulates malaria-induced immune responses primarily at early blood stage infection contributing to parasitemia control and resistance to mortality
- PMID: 28416609
- PMCID: PMC5454118
- DOI: 10.1074/jbc.M117.781294
CD36 receptor regulates malaria-induced immune responses primarily at early blood stage infection contributing to parasitemia control and resistance to mortality
Abstract
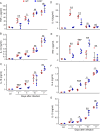
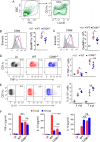
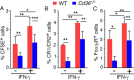
In malaria, CD36 plays several roles, including mediating parasite sequestration to host organs, phagocytic clearance of parasites, and regulation of immunity. Although the functions of CD36 in parasite sequestration and phagocytosis have been clearly defined, less is known about its role in malaria immunity. Here, to understand the function of CD36 in malaria immunity, we studied parasite growth, innate and adaptive immune responses, and host survival in WT and Cd36-/- mice infected with a non-lethal strain of Plasmodium yoelii Compared with Cd36-/- mice, WT mice had lower parasitemias and were resistant to death. At early but not at later stages of infection, WT mice had higher circulatory proinflammatory cytokines and lower anti-inflammatory cytokines than Cd36-/- mice. WT mice showed higher frequencies of proinflammatory cytokine-producing and lower frequencies of anti-inflammatory cytokine-producing dendritic cells (DCs) and natural killer cells than Cd36-/- mice. Cytokines produced by co-cultures of DCs from infected mice and ovalbumin-specific, MHC class II-restricted α/β (OT-II) T cells reflected CD36-dependent DC function. WT mice also showed increased Th1 and reduced Th2 responses compared with Cd36-/- mice, mainly at early stages of infection. Furthermore, in infected WT mice, macrophages and neutrophils expressed higher levels of phagocytic receptors and showed enhanced phagocytosis of parasite-infected erythrocytes than those in Cd36-/- mice in an IFN-γ-dependent manner. However, there were no differences in malaria-induced humoral responses between WT and Cd36-/- mice. Overall, the results show that CD36 plays a significant role in controlling parasite burden by contributing to proinflammatory cytokine responses by DCs and natural killer cells, Th1 development, phagocytic receptor expression, and phagocytic activity.
Keywords: CD36; Plasmodium yoelii; cellular immune response; cytokine induction; humoral response; immune regulation; malaria; phagocytic receptors; phagocytosis; resistance to disease.
© 2017 by The American Society for Biochemistry and Molecular Biology, Inc.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures






Similar articles
-
CD36 contributes to malaria parasite-induced pro-inflammatory cytokine production and NK and T cell activation by dendritic cells.PLoS One. 2013 Oct 28;8(10):e77604. doi: 10.1371/journal.pone.0077604. eCollection 2013. PLoS One. 2013. PMID: 24204889 Free PMC article.
-
Disruption of CD36 impairs cytokine response to Plasmodium falciparum glycosylphosphatidylinositol and confers susceptibility to severe and fatal malaria in vivo.J Immunol. 2007 Mar 15;178(6):3954-61. doi: 10.4049/jimmunol.178.6.3954. J Immunol. 2007. PMID: 17339496
-
Macrophage-mediated but gamma interferon-independent innate immune responses control the primary wave of Plasmodium yoelii parasitemia.Infect Immun. 2007 Dec;75(12):5806-18. doi: 10.1128/IAI.01005-07. Epub 2007 Oct 8. Infect Immun. 2007. PMID: 17923512 Free PMC article.
-
Dendritic cells, pro-inflammatory responses, and antigen presentation in a rodent malaria infection.Immunol Rev. 2004 Oct;201:35-47. doi: 10.1111/j.0105-2896.2004.00182.x. Immunol Rev. 2004. PMID: 15361231 Review.
-
Type I Interferons and Malaria: A Double-Edge Sword Against a Complex Parasitic Disease.Front Cell Infect Microbiol. 2020 Dec 2;10:594621. doi: 10.3389/fcimb.2020.594621. eCollection 2020. Front Cell Infect Microbiol. 2020. PMID: 33344264 Free PMC article. Review.
Cited by
-
Host genetics in malaria: lessons from mouse studies.Mamm Genome. 2018 Aug;29(7-8):507-522. doi: 10.1007/s00335-018-9744-9. Epub 2018 Mar 28. Mamm Genome. 2018. PMID: 29594458 Review.
-
The polymorphism analysis for CD36 among platelet donors.Sci Rep. 2024 Apr 12;14(1):8534. doi: 10.1038/s41598-024-58491-z. Sci Rep. 2024. PMID: 38609394 Free PMC article.
-
Circulating Monocytes, Tissue Macrophages, and Malaria.J Trop Med. 2019 Oct 2;2019:3720838. doi: 10.1155/2019/3720838. eCollection 2019. J Trop Med. 2019. PMID: 31662766 Free PMC article. Review.
-
Management challenge of a rare concomitant platelet glycoprotein IV/CD36 and IIb/IIIa deficiencies: Case illustration.Transfusion. 2025 Apr;65(4):767-772. doi: 10.1111/trf.18176. Epub 2025 Mar 3. Transfusion. 2025. PMID: 40033798 Free PMC article.
-
Skeleton binding protein-1-mediated parasite sequestration inhibits spontaneous resolution of malaria-associated acute respiratory distress syndrome.PLoS Pathog. 2021 Nov 29;17(11):e1010114. doi: 10.1371/journal.ppat.1010114. eCollection 2021 Nov. PLoS Pathog. 2021. PMID: 34843584 Free PMC article.
References
-
- World Health Organization (2015) World Malaria Report 2015, World Health Organization, Geneva
-
- Murray C. J., Rosenfeld L. C., Lim S. S., Andrews K. G., Foreman K. J., Haring D., Fullman N., Naghavi M., Lozano R., and Lopez A. D. (2012) Global malaria mortality between 1980 and 2010: a systematic analysis. Lancet 379, 413–431 - PubMed
-
- Battle K. E., Guerra C. A., Golding N., Duda K. A., Cameron E., Howes R. E., Elyazar I. R., Baird J. K., Reiner R. C. Jr., Gething P. W., Smith D. L., and Hay S. I. (2015) Global database of matched Plasmodium falciparum and P. vivax incidence and prevalence records from 1985–2013. Sci. Data 2, 150012. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

