Evidence for shear-mediated Ca2+ entry through mechanosensitive cation channels in human platelets and a megakaryocytic cell line
- PMID: 28416610
- PMCID: PMC5454102
- DOI: 10.1074/jbc.M116.766196
Evidence for shear-mediated Ca2+ entry through mechanosensitive cation channels in human platelets and a megakaryocytic cell line
Abstract
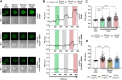
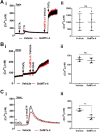
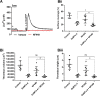
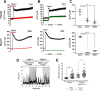
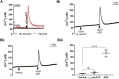
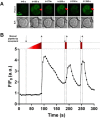
The role of mechanosensitive (MS) Ca2+-permeable ion channels in platelets is unclear, despite the importance of shear stress in platelet function and life-threatening thrombus formation. We therefore sought to investigate the expression and functional relevance of MS channels in human platelets. The effect of shear stress on Ca2+ entry in human platelets and Meg-01 megakaryocytic cells loaded with Fluo-3 was examined by confocal microscopy. Cells were attached to glass coverslips within flow chambers that allowed applications of physiological and pathological shear stress. Arterial shear (1002.6 s-1) induced a sustained increase in [Ca2+] i in Meg-01 cells and enhanced the frequency of repetitive Ca2+ transients by 80% in platelets. These Ca2+ increases were abrogated by the MS channel inhibitor Grammostola spatulata mechanotoxin 4 (GsMTx-4) or by chelation of extracellular Ca2+ Thrombus formation was studied on collagen-coated surfaces using DiOC6-stained platelets. In addition, [Ca2+] i and functional responses of washed platelet suspensions were studied with Fura-2 and light transmission aggregometry, respectively. Thrombus size was reduced 50% by GsMTx-4, independently of P2X1 receptors. In contrast, GsMTx-4 had no effect on collagen-induced aggregation or on Ca2+ influx via TRPC6 or Orai1 channels and caused only a minor inhibition of P2X1-dependent Ca2+ entry. The Piezo1 agonist, Yoda1, potentiated shear-dependent platelet Ca2+ transients by 170%. Piezo1 mRNA transcripts and protein were detected with quantitative RT-PCR and Western blotting, respectively, in both platelets and Meg-01 cells. We conclude that platelets and Meg-01 cells express the MS cation channel Piezo1, which may contribute to Ca2+ entry and thrombus formation under arterial shear.
Keywords: Piezo1; calcium; ion channel; mechanotransduction; platelet; shear stress.
© 2017 by The American Society for Biochemistry and Molecular Biology, Inc.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

