Hypoxia-inducible factor 1α activates insulin-induced gene 2 (Insig-2) transcription for degradation of 3-hydroxy-3-methylglutaryl (HMG)-CoA reductase in the liver
- PMID: 28416613
- PMCID: PMC5454117
- DOI: 10.1074/jbc.M117.788562
Hypoxia-inducible factor 1α activates insulin-induced gene 2 (Insig-2) transcription for degradation of 3-hydroxy-3-methylglutaryl (HMG)-CoA reductase in the liver
Abstract
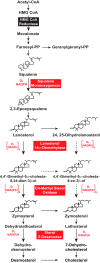
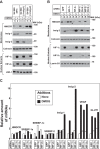
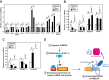
Cholesterol synthesis is a highly oxygen-consuming process. As such, oxygen deprivation (hypoxia) limits cholesterol synthesis through incompletely understood mechanisms mediated by the oxygen-sensitive transcription factor hypoxia-inducible factor 1α (HIF-1α). We show here that HIF-1α links pathways for oxygen sensing and feedback control of cholesterol synthesis in human fibroblasts by directly activating transcription of the INSIG-2 gene. Insig-2 is one of two endoplasmic reticulum membrane proteins that inhibit cholesterol synthesis by mediating sterol-induced ubiquitination and subsequent endoplasmic reticulum-associated degradation of the rate-limiting enzyme in the pathway, HMG-CoA reductase (HMGCR). Consistent with the results in cultured cells, hepatic levels of Insig-2 mRNA were enhanced in mouse models of hypoxia. Moreover, pharmacologic stabilization of HIF-1α in the liver stimulated HMGCR degradation via a reaction that requires the protein's prior ubiquitination and the presence of the Insig-2 protein. In summary, our results show that HIF-1α activates INSIG-2 transcription, leading to accumulation of Insig-2 protein, which binds to HMGCR and triggers its accelerated ubiquitination and degradation. These results indicate that HIF-mediated induction of Insig-2 and degradation of HMGCR are physiologically relevant events that guard against wasteful oxygen consumption and inappropriate cell growth during hypoxia.
Keywords: ER-associated degradation; cholesterol metabolism; endoplasmic reticulum (ER); hypoxia; isoprenoid.
© 2017 by The American Society for Biochemistry and Molecular Biology, Inc.
Conflict of interest statement
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health
Figures




References
-
- Goldstein J. L., DeBose-Boyd R. A., and Brown M. S. (2006) Protein sensors for membrane sterols. Cell 124, 35–46 - PubMed
-
- Sever N., Song B. L., Yabe D., Goldstein J. L., Brown M. S., and DeBose-Boyd R. A. (2003) Insig-dependent ubiquitination and degradation of mammalian 3-hydroxy-3-methylglutaryl-CoA reductase stimulated by sterols and geranylgeraniol. J. Biol. Chem. 278, 52479–52490 - PubMed
-
- Sever N., Yang T., Brown M. S., Goldstein J. L., and DeBose-Boyd R. A. (2003) Accelerated degradation of HMG CoA reductase mediated by binding of insig-1 to its sterol-sensing domain. Mol. Cell 11, 25–33 - PubMed
-
- Song B. L., Javitt N. B., and DeBose-Boyd R. A. (2005) Insig-mediated degradation of HMG CoA reductase stimulated by lanosterol, an intermediate in the synthesis of cholesterol. Cell Metab. 1, 179–189 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

