A Molecular Mechanism To Switch the Aryl Hydrocarbon Receptor from a Transcription Factor to an E3 Ubiquitin Ligase
- PMID: 28416634
- PMCID: PMC5472827
- DOI: 10.1128/MCB.00630-16
A Molecular Mechanism To Switch the Aryl Hydrocarbon Receptor from a Transcription Factor to an E3 Ubiquitin Ligase
Abstract
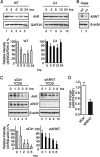
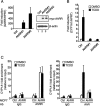
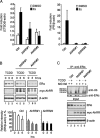
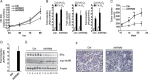
The aryl hydrocarbon receptor (AhR) is a ligand-activated transcription factor that is known as a mediator of toxic responses. Recently, it was shown that the AhR has dual functions. Besides being a transcription factor, it also possesses an intrinsic E3 ubiquitin ligase function that targets, e.g., the steroid receptors for proteasomal degradation. The aim of this study was to identify the molecular switch that determines whether the AhR acts as a transcription factor or an E3 ubiquitin ligase. To do this, we used the breast cancer cell line MCF7, which expresses a functional estrogen receptor alpha (ERα) signaling pathway. Our data suggest that aryl hydrocarbon receptor nuclear translocator (ARNT) plays an important role in the modulation of the dual functions of the AhR. ARNT knockdown dramatically impaired the transcriptional activation properties of the ligand-activated AhR but did not affect its E3 ubiquitin ligase function. The availability of ARNT itself is modulated by another basic helix-loop-helix (bHLH)-Per-ARNT-SIM (PAS) protein, the repressor of AhR function (AhRR). MCF7 cells overexpressing the AhRR showed lower ERα protein levels, reduced responsiveness to estradiol, and reduced growth rates. Importantly, when these cells were used to produce estrogen-dependent xenograft tumors in SCID mice, we also observed lower ERα protein levels and a reduced tumor mass, implying a tumor-suppressive-like function of the AhR in MCF7 xenograft tumors.
Keywords: E3 ubiquitin ligase; aryl hydrocarbon receptor; aryl hydrocarbon receptor nuclear translocator; aryl hydrocarbon receptor repressor; molecular switch; transcription factor.
Copyright © 2017 American Society for Microbiology.
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
