Autonomous sweat extraction and analysis applied to cystic fibrosis and glucose monitoring using a fully integrated wearable platform
- PMID: 28416667
- PMCID: PMC5422810
- DOI: 10.1073/pnas.1701740114
Autonomous sweat extraction and analysis applied to cystic fibrosis and glucose monitoring using a fully integrated wearable platform
Abstract
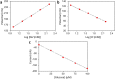
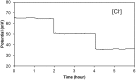
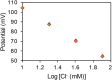
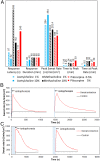
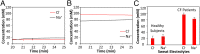
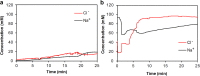
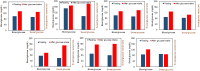
Perspiration-based wearable biosensors facilitate continuous monitoring of individuals' health states with real-time and molecular-level insight. The inherent inaccessibility of sweat in sedentary individuals in large volume (≥10 µL) for on-demand and in situ analysis has limited our ability to capitalize on this noninvasive and rich source of information. A wearable and miniaturized iontophoresis interface is an excellent solution to overcome this barrier. The iontophoresis process involves delivery of stimulating agonists to the sweat glands with the aid of an electrical current. The challenge remains in devising an iontophoresis interface that can extract sufficient amount of sweat for robust sensing, without electrode corrosion and burning/causing discomfort in subjects. Here, we overcame this challenge through realizing an electrochemically enhanced iontophoresis interface, integrated in a wearable sweat analysis platform. This interface can be programmed to induce sweat with various secretion profiles for real-time analysis, a capability which can be exploited to advance our knowledge of the sweat gland physiology and the secretion process. To demonstrate the clinical value of our platform, human subject studies were performed in the context of the cystic fibrosis diagnosis and preliminary investigation of the blood/sweat glucose correlation. With our platform, we detected the elevated sweat electrolyte content of cystic fibrosis patients compared with that of healthy control subjects. Furthermore, our results indicate that oral glucose consumption in the fasting state is followed by increased glucose levels in both sweat and blood. Our solution opens the possibility for a broad range of noninvasive diagnostic and general population health monitoring applications.
Keywords: biosensors; iontophoresis; noninvasive; personalized medicine; wearable.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
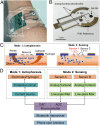

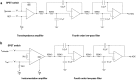

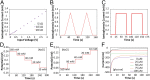
Similar articles
-
Flexible Electronics toward Wearable Sensing.Acc Chem Res. 2019 Mar 19;52(3):523-533. doi: 10.1021/acs.accounts.8b00500. Epub 2019 Feb 15. Acc Chem Res. 2019. PMID: 30767497 Review.
-
Fully integrated wearable sensor arrays for multiplexed in situ perspiration analysis.Nature. 2016 Jan 28;529(7587):509-514. doi: 10.1038/nature16521. Nature. 2016. PMID: 26819044 Free PMC article.
-
A Fully Integrated and Self-Powered Smartwatch for Continuous Sweat Glucose Monitoring.ACS Sens. 2019 Jul 26;4(7):1925-1933. doi: 10.1021/acssensors.9b00891. Epub 2019 Jul 4. ACS Sens. 2019. PMID: 31271034
-
Wearable Fluid Capture Devices for Electrochemical Sensing of Sweat.ACS Appl Mater Interfaces. 2019 Jan 9;11(1):238-243. doi: 10.1021/acsami.8b17419. Epub 2018 Dec 17. ACS Appl Mater Interfaces. 2019. PMID: 30516364
-
Wearable physiological systems and technologies for metabolic monitoring.J Appl Physiol (1985). 2018 Mar 1;124(3):548-556. doi: 10.1152/japplphysiol.00407.2017. Epub 2017 Sep 28. J Appl Physiol (1985). 2018. PMID: 28970200 Review.
Cited by
-
A Comprehensive Review of the Recent Developments in Wearable Sweat-Sensing Devices.Sensors (Basel). 2022 Oct 10;22(19):7670. doi: 10.3390/s22197670. Sensors (Basel). 2022. PMID: 36236769 Free PMC article. Review.
-
Molecularly selective nanoporous membrane-based wearable organic electrochemical device for noninvasive cortisol sensing.Sci Adv. 2018 Jul 20;4(7):eaar2904. doi: 10.1126/sciadv.aar2904. eCollection 2018 Jul. Sci Adv. 2018. PMID: 30035216 Free PMC article.
-
Flexible potentiometric pH sensors for wearable systems.RSC Adv. 2020 Feb 27;10(15):8594-8617. doi: 10.1039/d0ra00016g. eCollection 2020 Feb 27. RSC Adv. 2020. PMID: 35496561 Free PMC article. Review.
-
Nanofiber embedded bioinspired strong wet friction surface.Sci Adv. 2023 Oct 13;9(41):eadi4843. doi: 10.1126/sciadv.adi4843. Epub 2023 Oct 12. Sci Adv. 2023. PMID: 37824620 Free PMC article.
-
Wearable biosensors for healthcare monitoring.Nat Biotechnol. 2019 Apr;37(4):389-406. doi: 10.1038/s41587-019-0045-y. Epub 2019 Feb 25. Nat Biotechnol. 2019. PMID: 30804534 Free PMC article. Review.
References
-
- Kim DH, et al. Epidermal electronics. Science. 2011;333(6044):838–843. - PubMed
-
- Hammock ML, Chortos A, Tee BC, Tok JB, Bao Z. 25th anniversary article: The evolution of electronic skin (e-skin): A brief history, design considerations, and recent progress. Adv Mater. 2013;25(42):5997–6038. - PubMed
-
- Bandodkar AJ, Jeerapan I, Wang J. Wearable chemical sensors: Present challenges and future prospects. ACS Sens. 2016;1:464–482.
-
- Wang C, et al. User-interactive electronic skin for instantaneous pressure visualization. Nat Mater. 2013;12(10):899–904. - PubMed
-
- Xu S, et al. Soft microfluidic assemblies of sensors, circuits, and radios for the skin. Science. 2014;344(6179):70–74. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

