Selective in vivo removal of pathogenic anti-MAG autoantibodies, an antigen-specific treatment option for anti-MAG neuropathy
- PMID: 28416698
- PMCID: PMC5422814
- DOI: 10.1073/pnas.1619386114
Selective in vivo removal of pathogenic anti-MAG autoantibodies, an antigen-specific treatment option for anti-MAG neuropathy
Abstract
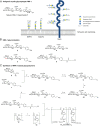
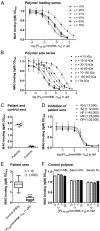
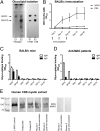
Anti-MAG (myelin-associated glycoprotein) neuropathy is a disabling autoimmune peripheral neuropathy caused by monoclonal IgM autoantibodies that recognize the carbohydrate epitope HNK-1 (human natural killer-1). This glycoepitope is highly expressed on adhesion molecules, such as MAG, present in myelinated nerve fibers. Because the pathogenicity and demyelinating properties of anti-MAG autoantibodies are well established, current treatments are aimed at reducing autoantibody levels. However, current therapies are primarily immunosuppressive and lack selectivity and efficacy. We therefore hypothesized that a significant improvement in the disease condition could be achieved by selectively neutralizing the pathogenic anti-MAG antibodies with carbohydrate-based ligands mimicking the natural HNK-1 glycoepitope 1. In an inhibition assay, a mimetic (2, mimHNK-1) of the natural HNK-1 epitope blocked the interaction of MAG with pathogenic IgM antibodies from patient sera but with only micromolar affinity. Therefore, considering the multivalent nature of the MAG-IgM interaction, polylysine polymers of different sizes were substituted with mimetic 2. With the most promising polylysine glycopolymer PL84(mimHNK-1)45 the inhibitory effect on patient sera could be improved by a factor of up to 230,000 per epitope, consequently leading to a low-nanomolar inhibitory potency. Because clinical studies indicate a correlation between the reduction of anti-MAG IgM levels and clinical improvement, an immunological surrogate mouse model for anti-MAG neuropathy producing high levels of anti-MAG IgM was developed. The observed efficient removal of these antibodies with the glycopolymer PL84(mimHNK-1)45 represents an important step toward an antigen-specific therapy for anti-MAG neuropathy.
Keywords: HNK-1 glycoepitope; IgM autoantibodies; demyelinating peripheral neuropathy; glycosylated polylysine; myelin-associated glycoprotein.
Conflict of interest statement
Conflict of interest statement. R.H., P.H., A.J.S., and B.E. are co-founders of a University of Basel spin-off, Polyneuron Pharmaceuticals AG, whose activity is related to the subject matter of this article. A.J.S. and B.E. are members of the advisory board, and B.E. is also a member of the board of directors. R.H., H.P., F.Y., A.J.S., and B.E. are named as co-inventors on relevant patent applications.
Figures

Similar articles
-
Selective inhibition of anti-MAG IgM autoantibody binding to myelin by an antigen-specific glycopolymer.J Neurochem. 2020 Sep;154(5):486-501. doi: 10.1111/jnc.15021. Epub 2020 Jun 23. J Neurochem. 2020. PMID: 32270492 Free PMC article.
-
The role of human natural killer-1 (HNK-1) carbohydrate in neuronal plasticity and disease.Biochim Biophys Acta Gen Subj. 2017 Oct;1861(10):2455-2461. doi: 10.1016/j.bbagen.2017.06.025. Epub 2017 Jul 12. Biochim Biophys Acta Gen Subj. 2017. PMID: 28709864 Review.
-
Anti-MAG neuropathy: From biology to clinical management.J Neuroimmunol. 2021 Dec 15;361:577725. doi: 10.1016/j.jneuroim.2021.577725. Epub 2021 Sep 28. J Neuroimmunol. 2021. PMID: 34610502 Review.
-
Anti-myelin associated glycoprotein antibodies recognize HNK-1 epitope on CNS.J Neuroimmunol. 2011 Jul;236(1-2):99-105. doi: 10.1016/j.jneuroim.2011.05.002. Epub 2011 May 31. J Neuroimmunol. 2011. PMID: 21621858
-
Antibodies to myelin-associated glycoprotein (anti-Mag) in IgM amyloidosis may influence expression of neuropathy in rare patients.Muscle Nerve. 2008 Apr;37(4):490-5. doi: 10.1002/mus.20955. Muscle Nerve. 2008. PMID: 18236455
Cited by
-
From Anti-EBV Immune Responses to the EBV Diseasome via Cross-reactivity.Glob Med Genet. 2020 Aug;7(2):51-63. doi: 10.1055/s-0040-1715641. Epub 2020 Aug 31. Glob Med Genet. 2020. PMID: 32939516 Free PMC article.
-
Selective Capture of Anti-N-glucosylated NTHi Adhesin Peptide Antibodies by a Multivalent Dextran Conjugate.Chembiochem. 2022 Feb 4;23(3):e202100515. doi: 10.1002/cbic.202100515. Epub 2021 Dec 6. Chembiochem. 2022. PMID: 34761861 Free PMC article.
-
Paraneoplastic Neuropathies: What's New Since the 2004 Recommended Diagnostic Criteria.Front Neurol. 2021 Oct 1;12:706169. doi: 10.3389/fneur.2021.706169. eCollection 2021. Front Neurol. 2021. PMID: 34659082 Free PMC article. Review.
-
Selective inhibition of anti-MAG IgM autoantibody binding to myelin by an antigen-specific glycopolymer.J Neurochem. 2020 Sep;154(5):486-501. doi: 10.1111/jnc.15021. Epub 2020 Jun 23. J Neurochem. 2020. PMID: 32270492 Free PMC article.
-
Individualized immunoglobulin therapy in chronic immune-mediated peripheral neuropathies.J Peripher Nerv Syst. 2018 Jun;23(2):78-87. doi: 10.1111/jns.12262. Epub 2018 Apr 19. J Peripher Nerv Syst. 2018. PMID: 29573033 Free PMC article. Review.
References
-
- Talamo G, Mir MA, Pandey MK, Sivik JK, Raheja D. IgM MGUS associated with anti-MAG neuropathy: A single institution experience. Ann Hematol. 2015;94:1011–1016. - PubMed
-
- Braun PE, Frail DE, Latov N. Myelin-associated glycoprotein is the antigen for a monoclonal IgM in polyneuropathy. J Neurochem. 1982;39:1261–1265. - PubMed
-
- Steck AJ, Murray N, Meier C, Page N, Perruisseau G. Demyelinating neuropathy and monoclonal IgM antibody to myelin-associated glycoprotein. Neurology. 1983;33:19–23. - PubMed
-
- Steck AJ, Stalder AK, Renaud S. Anti-myelin-associated glycoprotein neuropathy. Curr Opin Neurol. 2006;19:458–463. - PubMed
-
- Dalakas MC. Pathogenesis and treatment of anti-MAG neuropathy. Curr Treat Options Neurol. 2010;12:71–83. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

