Essential Neuroscience in Immunology
- PMID: 28416717
- PMCID: PMC5426063
- DOI: 10.4049/jimmunol.1601613
Essential Neuroscience in Immunology
Abstract
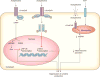
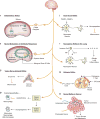
The field of immunology is principally focused on the molecular mechanisms by which hematopoietic cells initiate and maintain innate and adaptive immunity. That cornerstone of attention has been expanded by recent discoveries that neuronal signals occupy a critical regulatory niche in immunity. The discovery is that neuronal circuits operating reflexively regulate innate and adaptive immunity. One particularly well-characterized circuit regulating innate immunity, the inflammatory reflex, is dependent upon action potentials transmitted to the reticuloendothelial system via the vagus and splenic nerves. This field has grown significantly with the identification of several other reflexes regulating discrete immune functions. As outlined in this review, the delineation of these mechanisms revealed a new understanding of immunity, enabled a first-in-class clinical trial using bioelectronic devices to inhibit cytokines and inflammation in rheumatoid arthritis patients, and provided a mosaic view of immunity as the integration of hematopoietic and neural responses to infection and injury.
Copyright © 2017 by The American Association of Immunologists, Inc.
Figures
References
-
- Burke RE. Sir Charles Sherrington’s the integrative action of the nervous system: A centenary appreciation. Brain. 2007;130:887–894. - PubMed
-
- Lesnikov VA, Efremov OM, Korneva EA, Van Damme J, Billiau A. Fever produced by intrahypothalamic injection of interleukin-1 and interleukin-6. Cytokine. 1991;3:195–198. - PubMed
-
- Dinarello Ca. Cytokines as endogenous pyrogens. J Infect Dis. 1999;179(Suppl):S294–S304. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

