The angiotensin receptor blocker, Losartan, inhibits mammary tumor development and progression to invasive carcinoma
- PMID: 28416734
- PMCID: PMC5386636
- DOI: 10.18632/oncotarget.15553
The angiotensin receptor blocker, Losartan, inhibits mammary tumor development and progression to invasive carcinoma
Abstract
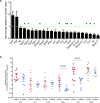
Drugs that target the Renin-Angiotensin System (RAS) have recently come into focus for their potential utility as cancer treatments. The use of Angiotensin Receptor Blockers (ARBs) and Angiotensin-Converting Enzyme (ACE) Inhibitors (ACEIs) to manage hypertension in cancer patients is correlated with improved survival outcomes for renal, prostate, breast and small cell lung cancer. Previous studies demonstrate that the Angiotensin Receptor Type I (AT1R) is linked to breast cancer pathogenesis, with unbiased analysis of gene-expression studies identifying significant up-regulation of AGTR1, the gene encoding AT1R in ER+ve/HER2-ve tumors correlating with poor prognosis. However, there is no evidence, so far, of the functional contribution of AT1R to breast tumorigenesis. We explored the potential therapeutic benefit of ARB in a carcinogen-induced mouse model of breast cancer and clarified the mechanisms associated with its success.Mammary tumors were induced with 7,12-dimethylbenz[α]antracene (DMBA) and medroxyprogesterone acetate (MPA) in female wild type mice and the effects of the ARB, Losartan treatment assessed in a preventative setting (n = 15 per group). Tumor histopathology was characterised by immunohistochemistry, real-time qPCR to detect gene expression signatures, and tumor cytokine levels measured with quantitative bioplex assays. AT1R was detected with radiolabelled ligand binding assays in fresh frozen tumor samples.We showed that therapeutic inhibition of AT1R, with Losartan, resulted in a significant reduction in tumor burden; and no mammary tumor incidence in 20% of animals. We observed a significant reduction in tumor progression from DCIS to invasive cancer with Losartan treatment. This was associated with reduced tumor cell proliferation and a significant reduction in IL-6, pSTAT3 and TNFα levels. Analysis of tumor immune cell infiltrates, however, demonstrated no significant differences in the recruitment of lymphocytes or tumour-associated macrophages in Losartan or vehicle-treated mammary tumors.Analysis of AT1R expression with radiolabelled ligand binding assays in human breast cancer biopsies showed high AT1R levels in 30% of invasive ductal carcinomas analysed. Furthermore, analysis of the TCGA database identified that high AT1R expression to be associated with luminal breast cancer subtype.Our in vivo data and analysis of human invasive ductal carcinoma samples identify the AT1R is a potential therapeutic target in breast cancer, with the availability of a range of well-tolerated inhibitors currently used in clinics. We describe a novel signalling pathway critical in breast tumorigenesis, that may provide new therapeutic avenues to complement current treatments.
Keywords: angiotensin receptor; interleukin 6; invasive ductal carcinoma; luminal breast cancer; tumor necrosis factor.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures




Comment in
-
Why isn't the angiotensin type 1 receptor a target in cancer?Oncotarget. 2017 Mar 21;8(12):18618-18619. doi: 10.18632/oncotarget.15682. Oncotarget. 2017. PMID: 28423631 Free PMC article. No abstract available.
Similar articles
-
Role of Angiotensin II type 1 receptor on renal NAD(P)H oxidase, oxidative stress and inflammation in nitric oxide inhibition induced-hypertension.Life Sci. 2015 Mar 1;124:81-90. doi: 10.1016/j.lfs.2015.01.005. Epub 2015 Jan 24. Life Sci. 2015. PMID: 25623850 Free PMC article.
-
Angiotensin II receptor type 1 blockers suppress the cell proliferation effects of angiotensin II in breast cancer cells by inhibiting AT1R signaling.Oncol Rep. 2012 Jun;27(6):1893-903. doi: 10.3892/or.2012.1720. Epub 2012 Mar 13. Oncol Rep. 2012. PMID: 22426690
-
Perindopril, fosinopril and losartan inhibited the progression of diethylnitrosamine-induced hepatocellular carcinoma in mice via the inactivation of nuclear transcription factor kappa-B.Toxicol Lett. 2018 Oct 1;295:32-40. doi: 10.1016/j.toxlet.2018.05.036. Epub 2018 May 31. Toxicol Lett. 2018. PMID: 29859236
-
Role of the renin-angiotensin system in gynecologic cancers.Curr Cancer Drug Targets. 2011 May;11(4):405-11. doi: 10.2174/156800911795538057. Curr Cancer Drug Targets. 2011. PMID: 21395551 Review.
-
Angiotensin 2 type 1 receptor blockade different affects postishemic kidney injury in normotensive and hypertensive rats.J Physiol Biochem. 2016 Dec;72(4):813-820. doi: 10.1007/s13105-016-0514-4. Epub 2016 Aug 18. J Physiol Biochem. 2016. PMID: 27534651 Review.
Cited by
-
Biocompatible PEO-b-PCL Nanosized Micelles as Drug Carriers: Structure and Drug-Polymer Interactions.Nanomaterials (Basel). 2020 Sep 18;10(9):1872. doi: 10.3390/nano10091872. Nanomaterials (Basel). 2020. PMID: 32962043 Free PMC article.
-
Repurposing of Chronically Used Drugs in Cancer Therapy: A Chance to Grasp.Cancers (Basel). 2023 Jun 15;15(12):3199. doi: 10.3390/cancers15123199. Cancers (Basel). 2023. PMID: 37370809 Free PMC article. Review.
-
Losartan improves the therapeutic effect of metronomic cyclophosphamide in triple negative mammary cancer models.Oncotarget. 2020 Aug 11;11(32):3048-3060. doi: 10.18632/oncotarget.27694. eCollection 2020 Aug 11. Oncotarget. 2020. PMID: 32850009 Free PMC article.
-
The Network of Angiotensin Receptors in Breast Cancer.Cells. 2020 May 27;9(6):1336. doi: 10.3390/cells9061336. Cells. 2020. PMID: 32471115 Free PMC article. Review.
-
Modifying the Tumour Microenvironment: Challenges and Future Perspectives for Anticancer Plasma Treatments.Cancers (Basel). 2019 Dec 2;11(12):1920. doi: 10.3390/cancers11121920. Cancers (Basel). 2019. PMID: 31810265 Free PMC article. Review.
References
-
- Keizman D, Huang P, Eisenberger MA, Pili R, Kim JJ, Antonarakis ES, Hammers H, Carducci MA. Angiotensin system inhibitors and outcome of sunitinib treatment in patients with metastatic renal cell carcinoma: a retrospective examination. Eur J Cancer. 2011;47:1955–61. doi: 10.1016/j.ejca.2011.04.019. - DOI - PMC - PubMed
-
- Nakai Y, Isayama H, Ijichi H, Sasaki T, Sasahira N, Hirano K, Kogure H, Kawakubo K, Yagioka H, Yashima Y, Mizuno S, Yamamoto K, Arizumi T, et al. Inhibition of renin-angiotensin system affects prognosis of advanced pancreatic cancer receiving gemcitabine. Br J Cancer. 2010;103:1644–8. doi: 10.1038/sj.bjc.6605955. - DOI - PMC - PubMed
-
- Wilop S, von Hobe S, Crysandt M, Esser A, Osieka R, Jost E. Impact of angiotensin I converting enzyme inhibitors and angiotensin II type 1 receptor blockers on survival in patients with advanced non-small-cell lung cancer undergoing first-line platinum-based chemotherapy. J Cancer Res Clin Oncol. 2009;135:1429–35. doi: 10.1007/s00432-009-0587-3. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

