Neutrophil stunning by metoprolol reduces infarct size
- PMID: 28416795
- PMCID: PMC5399300
- DOI: 10.1038/ncomms14780
Neutrophil stunning by metoprolol reduces infarct size
Abstract
The β1-adrenergic-receptor (ADRB1) antagonist metoprolol reduces infarct size in acute myocardial infarction (AMI) patients. The prevailing view has been that metoprolol acts mainly on cardiomyocytes. Here, we demonstrate that metoprolol reduces reperfusion injury by targeting the haematopoietic compartment. Metoprolol inhibits neutrophil migration in an ADRB1-dependent manner. Metoprolol acts during early phases of neutrophil recruitment by impairing structural and functional rearrangements needed for productive engagement of circulating platelets, resulting in erratic intravascular dynamics and blunted inflammation. Depletion of neutrophils, ablation of Adrb1 in haematopoietic cells, or blockade of PSGL-1, the receptor involved in neutrophil-platelet interactions, fully abrogated metoprolol's infarct-limiting effects. The association between neutrophil count and microvascular obstruction is abolished in metoprolol-treated AMI patients. Metoprolol inhibits neutrophil-platelet interactions in AMI patients by targeting neutrophils. Identification of the relevant role of ADRB1 in haematopoietic cells during acute injury and the protective role upon its modulation offers potential for developing new therapeutic strategies.
Conflict of interest statement
The authors declare no competing financial interests.
Figures
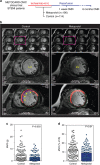
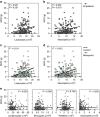


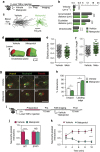


Comment in
-
Stunning neutrophils changes the forecast: No more showers.Sci Transl Med. 2017 May 3;9(388):eaan3775. doi: 10.1126/scitranslmed.aan3775. Sci Transl Med. 2017. PMID: 28469035
References
-
- Ibanez B., Heusch G., Ovize M. & Van de Werf F. Evolving therapies for myocardial ischemia/reperfusion injury. J. Am. Coll. Cardiol. 65, 1454–1471 (2015). - PubMed
-
- Vinten-Johansen J. Involvement of neutrophils in the pathogenesis of lethal myocardial reperfusion injury. Cardiovasc. Res. 61, 481–497 (2004). - PubMed
-
- Kolaczkowska E. & Kubes P. Neutrophil recruitment and function in health and inflammation. Nat. Rev. Immunol. 13, 159–175 (2013). - PubMed
-
- Fernandez-Jimenez R. et al.. Pathophysiology underlying the bimodal edema phenomenon after myocardial ischemia/reperfusion. J. Am. Coll. Cardiol. 66, 816–828 (2015). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

