A new inhibitor of the β-arrestin/AP2 endocytic complex reveals interplay between GPCR internalization and signalling
- PMID: 28416805
- PMCID: PMC5399295
- DOI: 10.1038/ncomms15054
A new inhibitor of the β-arrestin/AP2 endocytic complex reveals interplay between GPCR internalization and signalling
Abstract
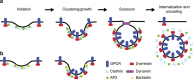
In addition to G protein-coupled receptor (GPCR) desensitization and endocytosis, β-arrestin recruitment to ligand-stimulated GPCRs promotes non-canonical signalling cascades. Distinguishing the respective contributions of β-arrestin recruitment to the receptor and β-arrestin-promoted endocytosis in propagating receptor signalling has been limited by the lack of selective analytical tools. Here, using a combination of virtual screening and cell-based assays, we have identified a small molecule that selectively inhibits the interaction between β-arrestin and the β2-adaptin subunit of the clathrin adaptor protein AP2 without interfering with the formation of receptor/β-arrestin complexes. This selective β-arrestin/β2-adaptin inhibitor (Barbadin) blocks agonist-promoted endocytosis of the prototypical β2-adrenergic (β2AR), V2-vasopressin (V2R) and angiotensin-II type-1 (AT1R) receptors, but does not affect β-arrestin-independent (transferrin) or AP2-independent (endothelin-A) receptor internalization. Interestingly, Barbadin fully blocks V2R-stimulated ERK1/2 activation and blunts cAMP accumulation promoted by both V2R and β2AR, supporting the concept of β-arrestin/AP2-dependent signalling for both G protein-dependent and -independent pathways.
Conflict of interest statement
The authors declare no competing financial interests.
Figures


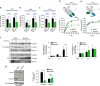

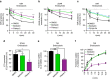
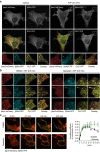
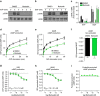
References
-
- Lohse M. J., Benovic J. L., Codina J., Caron M. G. & Lefkowitz R. J. Beta-Arrestin: a protein that regulates beta-adrenergic receptor function. Science 248, 1547–1550 (1990). - PubMed
-
- Ferguson S. S. et al. Role of beta-arrestin in mediating agonist-promoted G protein-coupled receptor internalization. Science 271, 363–366 (1996). - PubMed
-
- Goodman O. B. Jr et al. Beta-arrestin acts as a clathrin adaptor in endocytosis of the beta2-adrenergic receptor. Nature 383, 447–450 (1996). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

