Perisomatic changes in h-channels regulate depressive behaviors following chronic unpredictable stress
- PMID: 28416809
- PMCID: PMC5647208
- DOI: 10.1038/mp.2017.28
Perisomatic changes in h-channels regulate depressive behaviors following chronic unpredictable stress
Abstract
Chronic stress can be a precipitating factor in the onset of depression. Lentiviral-mediated knockdown of HCN1 protein expression and reduction of functional Ih produce antidepressant behavior. However, whether h-channels are altered in an animal model of depression is not known. We found that perisomatic HCN1 protein expression and Ih-sensitive physiological measurements were significantly increased in dorsal but not in ventral CA1 region/neurons following chronic unpredictable stress (CUS), a widely accepted model for major depressive disorder. Cell-attached patch clamp recordings confirmed that perisomatic Ih was increased in dorsal CA1 neurons following CUS. Furthermore, when dorsal CA1 Ih was reduced by shRNA-HCN1, the CUS-induced behavioral deficits were prevented. Finally, rats infused in the dorsal CA1 region with thapsigargin, an irreversible inhibitor of the SERCA pump, exhibited anxiogenic-like behaviors and increased Ih, similar to that observed following CUS. Our results suggest that CUS, but not acute stress, leads to an increase in perisomatic Ih in dorsal CA1 neurons and that HCN channels represent a potential target for the treatment of major depressive disorder.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


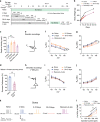

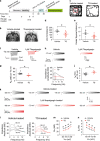
Comment in
-
A Possible Link Between HCN Channels and Depression.Chronic Stress (Thousand Oaks). 2018 Jan-Dec;2:2470547018787781. doi: 10.1177/2470547018787781. Epub 2018 Jul 24. Chronic Stress (Thousand Oaks). 2018. PMID: 30259006 Free PMC article.
Similar articles
-
Glucocorticoid-glucocorticoid receptor-HCN1 channels reduce neuronal excitability in dorsal hippocampal CA1 neurons.Mol Psychiatry. 2022 Oct;27(10):4035-4049. doi: 10.1038/s41380-022-01682-9. Epub 2022 Jul 15. Mol Psychiatry. 2022. PMID: 35840797 Free PMC article.
-
Protein kinase C bidirectionally modulates Ih and hyperpolarization-activated cyclic nucleotide-gated (HCN) channel surface expression in hippocampal pyramidal neurons.J Physiol. 2015 Jul 1;593(13):2779-92. doi: 10.1113/JP270453. Epub 2015 May 22. J Physiol. 2015. PMID: 25820761 Free PMC article.
-
Hyperpolarization-Activated Channel 1 Modulates Resilience and Susceptibility to Social Avoidance Induced by Witnessing Social Defeat Stress.Biol Psychiatry. 2025 Sep 1;98(5):378-393. doi: 10.1016/j.biopsych.2025.03.025. Epub 2025 Apr 8. Biol Psychiatry. 2025. PMID: 40210080
-
The Impact of Altered HCN1 Expression on Brain Function and Its Relationship with Epileptogenesis.Curr Neuropharmacol. 2023;21(10):2070-2078. doi: 10.2174/1570159X21666230214110333. Curr Neuropharmacol. 2023. PMID: 37366350 Free PMC article. Review.
-
Hyperpolarization-activated and cyclic nucleotide-gated channel proteins as emerging new targets in neuropathic pain.Rev Neurosci. 2019 Jul 26;30(6):639-649. doi: 10.1515/revneuro-2018-0094. Rev Neurosci. 2019. PMID: 30768426 Review.
Cited by
-
Targeting PDK2 rescues stress-induced impaired brain energy metabolism.Mol Psychiatry. 2023 Oct;28(10):4138-4150. doi: 10.1038/s41380-023-02098-9. Epub 2023 May 15. Mol Psychiatry. 2023. PMID: 37188779
-
Altered synaptic homeostasis: a key factor in the pathophysiology of depression.Cell Biosci. 2025 Feb 25;15(1):29. doi: 10.1186/s13578-025-01369-y. Cell Biosci. 2025. PMID: 40001206 Free PMC article. Review.
-
Antidepressant-like activity of a brain penetrant HCN channel inhibitor in mice.Front Pharmacol. 2023 May 10;14:1159527. doi: 10.3389/fphar.2023.1159527. eCollection 2023. Front Pharmacol. 2023. PMID: 37234718 Free PMC article.
-
Inhibition of HCN1 currents by norquetiapine, an active metabolite of the atypical anti-psychotic drug quetiapine.Front Pharmacol. 2024 Oct 7;15:1445509. doi: 10.3389/fphar.2024.1445509. eCollection 2024. Front Pharmacol. 2024. PMID: 39434909 Free PMC article.
-
Interaction Between HCN and Slack Channels Regulates mPFC Pyramidal Cell Excitability in Working Memory Circuits.Mol Neurobiol. 2024 Apr;61(4):2430-2445. doi: 10.1007/s12035-023-03719-8. Epub 2023 Oct 27. Mol Neurobiol. 2024. PMID: 37889366
References
-
- Campbell S, Marriott M, Nahmias C, MacQueen GM. Lower hippocampal volume in patients suffering from depression: a meta-analysis. Am J Psychiatry 2004; 161: 598–607. - PubMed
-
- Kennedy SH, Evans KR, Kruger S, Mayberg HS, Meyer JH, McCann S et al. Changes in regional brain glucose metabolism measured with positron emission tomography after paroxetine treatment of major depression. Am J Psychiatry 2001; 158: 899–905. - PubMed
-
- Mayberg HS, Brannan SK, Tekell JL, Silva JA, Mahurin RK, McGinnis S et al. Regional metabolic effects of fluoxetine in major depression: serial changes and relationship to clinical response. Biol Psychiatry 2000; 48: 830–843. - PubMed
-
- Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S et al. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science 2003; 301: 805–809. - PubMed
-
- Nestler EJ, Barrot M, DiLeone RJ, Eisch AJ, Gold SJ, Monteggia LM. Neurobiology of depression. Neuron 2002; 34: 13–25. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

