Regulation of the glucose supply from capillary to tissue examined by developing a capillary model
- PMID: 28417297
- PMCID: PMC10717424
- DOI: 10.1007/s12576-017-0538-8
Regulation of the glucose supply from capillary to tissue examined by developing a capillary model
Abstract
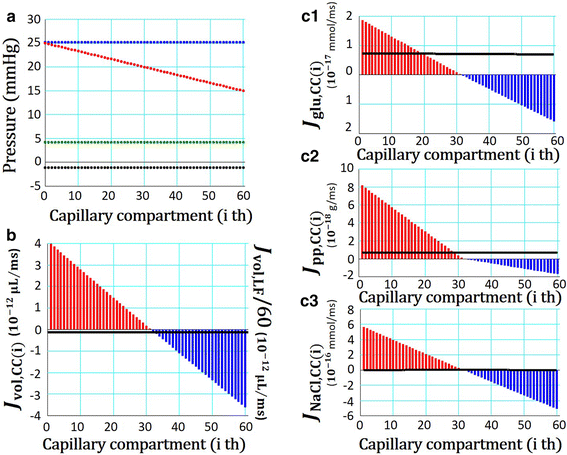
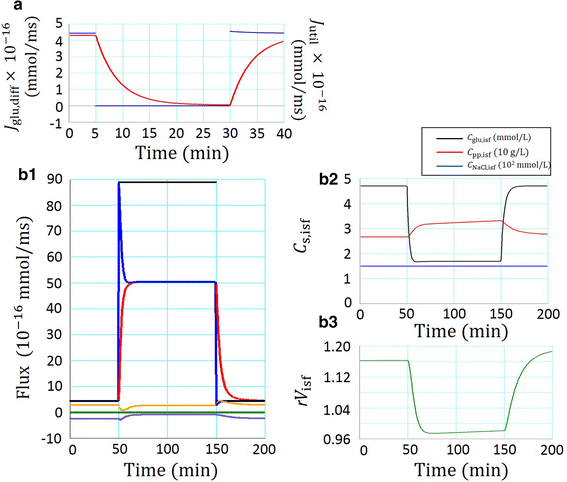
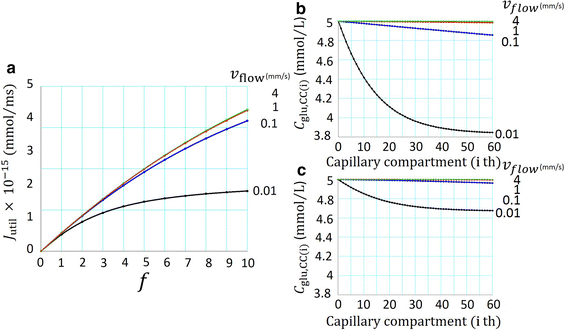
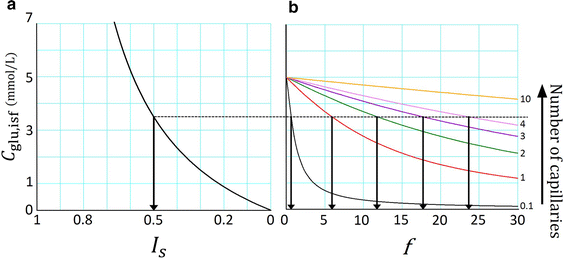
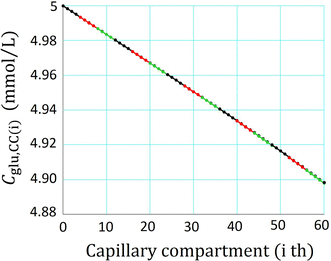
A new glucose transport model relying upon diffusion and convection across the capillary membrane was developed, and supplemented with tissue space and lymph flow. The rate of glucose utilization (J util) in the tissue space was described as a saturation function of glucose concentration in the interstitial fluid (C glu,isf), and was varied by applying a scaling factor f to J max. With f = 0, the glucose diffusion ceased within ~20 min. While, with increasing f, the diffusion was accelerated through a decrease in C glu,isf, but the convective flux remained close to resting level. When the glucose supplying capacity of the capillary was measured with a criterion of J util /J max = 0.5, the capacity increased in proportion to the number of perfused capillaries. A consistent profile of declining C glu,isf along the capillary axis was observed at the criterion of 0.5 irrespective of the capillary number. Increasing blood flow scarcely improved the supplying capacity.
Keywords: Convective glucose flux; Diffusion across the capillary membrane; Glucose supplying capacity; Mathematical capillary model; Reflection coefficient.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures



Similar articles
-
Convective diffusion of nanoparticles from the epithelial barrier toward regional lymph nodes.Adv Colloid Interface Sci. 2013 Nov;199-200:23-43. doi: 10.1016/j.cis.2013.06.002. Epub 2013 Jun 10. Adv Colloid Interface Sci. 2013. PMID: 23859221 Free PMC article. Review.
-
Mode of transcapillary transport of insulin and insulin analog NN304 in dog hindlimb: evidence for passive diffusion.Diabetes. 2002 Mar;51(3):574-82. doi: 10.2337/diabetes.51.3.574. Diabetes. 2002. PMID: 11872653
-
Role of villus microcirculation in intestinal absorption of glucose: coupling of epithelial with endothelial transport.J Physiol. 2003 Dec 1;553(Pt 2):561-74. doi: 10.1113/jphysiol.2003.043257. Epub 2003 Aug 22. J Physiol. 2003. PMID: 12937296 Free PMC article.
-
A model of nitric oxide capillary exchange.Microcirculation. 2003 Dec;10(6):479-95. doi: 10.1038/sj.mn.7800210. Microcirculation. 2003. PMID: 14745461
-
A mathematical model of interstitial transport. II. Microvascular exchange in mesentery.Microvasc Res. 1990 May;39(3):279-306. doi: 10.1016/0026-2862(90)90043-q. Microvasc Res. 1990. PMID: 2194092 Review.
Cited by
-
Relating Macroscopic PET Radiomics Features to Microscopic Tumor Phenotypes Using a Stochastic Mathematical Model of Cellular Metabolism and Proliferation.Cancers (Basel). 2024 Jun 13;16(12):2215. doi: 10.3390/cancers16122215. Cancers (Basel). 2024. PMID: 38927921 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

