Prophylactic anti-staphylococcal antibiotics for cystic fibrosis
- PMID: 28417451
- PMCID: PMC6478147
- DOI: 10.1002/14651858.CD001912.pub4
Prophylactic anti-staphylococcal antibiotics for cystic fibrosis
Update in
-
Prophylactic anti-staphylococcal antibiotics for cystic fibrosis.Cochrane Database Syst Rev. 2020 Sep 30;9(9):CD001912. doi: 10.1002/14651858.CD001912.pub5. Cochrane Database Syst Rev. 2020. PMID: 32997797 Free PMC article.
Abstract
Background: Staphylococcus aureus causes pulmonary infection in young children with cystic fibrosis. Prophylactic antibiotics are prescribed hoping to prevent such infection and lung damage. Antibiotics have adverse effects and long-term use might lead to infection with Pseudomonas aeruginosa. This is an update of a previously published review.
Objectives: To assess continuous oral antibiotic prophylaxis to prevent the acquisition of Staphylococcus aureus versus no prophylaxis in people with cystic fibrosis, we tested these hypotheses. Prophylaxis:1. improves clinical status, lung function and survival;2. causes adverse effects (e.g. diarrhoea, skin rash, candidiasis);3. leads to fewer isolates of common pathogens from respiratory secretions;4. leads to the emergence of antibiotic resistance and colonisation of the respiratory tract with Pseudomonas aeruginosa.
Search methods: We searched the Cochrane Cystic Fibrosis and Genetic Disorders Group Trials Register, comprising references identified from comprehensive electronic database searches, handsearches of relevant journals and abstract books of conference proceedings. Companies manufacturing anti-staphylococcal antibiotics were contacted.Most recent search of the Group's Register: 29 September 2016.
Selection criteria: Randomised trials of continuous oral prophylactic antibiotics (given for at least one year) compared to intermittent antibiotics given 'as required', in people with cystic fibrosis of any disease severity.
Data collection and analysis: The authors assessed studies for eligibility and methodological quality and extracted data.
Main results: We included four studies, with a total of 401 randomised participants aged zero to seven years on enrolment; one study is ongoing. The two older included studies generally had a higher risk of bias across all domains, but in particular due to a lack of blinding and incomplete outcome data, than the two more recent studies. We only regarded the most recent study as being generally free of bias, although even here we were not certain of the effect of the per protocol analysis on the study results. Evidence was downgraded based on GRADE assessments and outcome results ranged from moderate to low quality. Downgrading decisions were due to limitations in study design (all outcomes); for imprecision (number of people needing additional antibiotics); and for inconsistency (weight z score).Fewer children receiving anti-staphylococcal antibiotic prophylaxis had one or more isolates of Staphylococcus aureus (low quality evidence). There was no significant difference between groups in infant or conventional lung function (moderate quality evidence). We found no significant effect on nutrition (low quality evidence), hospital admissions, additional courses of antibiotics (low quality evidence) or adverse effects (moderate quality evidence). There was no significant difference in the number of isolates of Pseudomonas aeruginosa between groups (low quality evidence), though there was a trend towards a lower cumulative isolation rate of Pseudomonas aeruginosa in the prophylaxis group at two and three years and towards a higher rate from four to six years. As the studies reviewed lasted six years or less, conclusions cannot be drawn about the long-term effects of prophylaxis.
Authors' conclusions: Anti-staphylococcal antibiotic prophylaxis leads to fewer children having isolates of Staphylococcus aureus, when commenced early in infancy and continued up to six years of age. The clinical importance of this finding is uncertain. Further research may establish whether the trend towards more children with CF with Pseudomonas aeruginosa, after four to six years of prophylaxis, is a chance finding and whether choice of antibiotic or duration of treatment might influence this.
Conflict of interest statement
ARS declares relevant activities of membership of a MPEX steering committee, advisory board member (Vertex, Gilead and MPEX), lectures paid for by Gilead and Novartis.
MR declares no known conflict of interest.
Clarification statement added from Alan Smyth, Co‐ordinating Editor on 19 February 2020: This review was found by the Cochrane Funding Arbiters, post‐publication, to be noncompliant with the
Figures






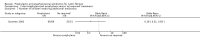





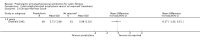
Update of
-
Prophylactic anti-staphylococcal antibiotics for cystic fibrosis.Cochrane Database Syst Rev. 2014 Nov 24;(11):CD001912. doi: 10.1002/14651858.CD001912.pub3. Cochrane Database Syst Rev. 2014. Update in: Cochrane Database Syst Rev. 2017 Apr 18;4:CD001912. doi: 10.1002/14651858.CD001912.pub4. PMID: 25419599 Updated.
References
References to studies included in this review
Chatfield 1991 {published and unpublished data}
-
- Owen G, West J, Maguire S, Ryley H, Goodchild M, Weller PH. Continuous and intermittent antibiotic therapy in cystic fibrosis patients to age 4 years [abstract]. Proceedings of the 17th European Cystic Fibrosis Conference; 1991 June 18‐21; Copenhagen, Denmark. 1991:95.
-
- West J, Smith AW, Brown MRW, Weller PH. Longitudinal relationship between clinical status, lung infection and immune responses in young cystic fibrosis patients [abstract]. Pediatric Pulmonology 1990;Suppl 5:222.
-
- Williams J, Alfaham M, Ryley HC, Goodchild MC, Weller PH, Dodge JA. Screening for cystic fibrosis in Wales and the West Midlands 2: Clinical evaluation [abstract]. Excerpta Medica, Asia Pacific Congress Series 1988;74:G(b)3.
Schlesinger 1984 {unpublished data only}
-
- Schlesinger E, Muller W, Hardt H, Schirg E, Rieger CHL. Effect of long‐term continuous anti‐staphylococcal antibiotic treatment in young children with cystic fibrosis [abstract]. Proceedings of the 9th International Cystic Fibrosis Congress. 1984:4.14.
Stutman 2002 {published and unpublished data}
-
- Stutman HR, Lieberman JM, Nussbaum E, Marks MI. Antibiotic prophylaxis in infants and young children with cystic fibrosis: A randomised controlled trial. Journal of Pediatrics 2002;140(3):299‐305. - PubMed
-
- Stutman HR, Marks MI. Cephalexin prophylaxis in newly diagnosed infants with cystic fibrosis [abstract]. Proceedings of the 6th Annual North American Cystic Fibrosis Conference; 1992. 1992:147‐8.
Weaver 1994 {published and unpublished data}
-
- Weaver LT, Green MR, Nicholson K, Heeley AF, Mills J, Kuzemko JA. Continuous prophylactic flucloxacillin improves outcome of infants with cystic fibrosis (CF) detected soon after birth [abstract]. Proceedings of 63rd Annual Meeting of the British Paediatric Association; 1991; Warwick. 1991:24.
References to studies excluded from this review
Ballestero 1992 {published data only}
-
- Ballestero S, Villaverde R, Escobar H, Baquero F. Susceptibility to various anti‐microbial agents of Staphylococcus aureus isolates from cystic fibrosis patients. European Journal of Clinical Microbiology & Infectious Diseases 1992;11(12):1193‐4. - PubMed
Brown 1980 {published data only}
-
- Brown J. Efficacy of antimicrobial drugs against staphylococci in cystic fibrosis. Australian Paediatric Journal 1980;16(3):207‐9. - PubMed
Denning 1977 {unpublished data only}
-
- Denning CR, Park S, Grece CA, Mellin GW. Continuous versus intermittent oral antibiotics in the management of patients with cystic fibrosis [abstract]. 18th Annual Meeting Cystic Fibrosis Club Abstracts. 1977:23.
Feigelson 1993 {published data only}
-
- Feigelson J, Pecau Y. Fusidic acid in cystic fibrosis [Action therapeutique de l'acide fusidique dans la mucoviscidose]. Pediatre 1993;29(138):111‐3.
Harrison 1985 {published data only}
-
- Harrison CJ, Marks MI, Welch DF, Sharma BB, Baker D, Dice J. A multicentric comparison of related pharmacologic features of cephalexin and dicloxacillin given for two months to young children with cystic fibrosis. Pediatric Pharmacology 1985;5(1):7‐16. - PubMed
Jensen 1990 {published data only}
-
- Jensen T, Lanng S, Faber M, Rosdahl VT, Hoiby N, Koch C. Clinical experiences with fusidic acid in cystic fibrosis patients. Journal of Antimicrobial Chemotherapy 1990;25(Suppl B):45‐52. - PubMed
Keel 2011 {published data only}
Keller 2010 {published data only}
-
- Keller M, Coates AL, Griese M, Denk O, Schierholz J, Knoch M. In‐vivo data support equivalent therapeutic efficacy of a new tobramycin inhalation solution (150mg/1.5ml) administered by the eFlow® electronic nebuliser compared to TOBI® in the PARI LC PLUS® [abstract]. Journal of Cystic Fibrosis 2010;9(Suppl 1):S22, Abstract no: 84. [CFGD Register: PI241]
Kerrebijn 1984 {published data only}
-
- Kerrebijn KF. Prospective study on the effect of daily and intermittent antibiotic treatment in cystic fibrosis. In: Lawson D editor(s). CF: horizons. Chichester: John Wiley and Son, 1984:273.
Loening‐Baucke 1979 {published data only}
-
- Loening‐Baucke V, Mischler E, Myers MG. A placebo controlled trial of cephalexin therapy in the ambulatory management of patients with cystic fibrosis. Journal of Pediatrics 1979;95(4):630‐7. - PubMed
-
- Loening‐Baucke V, Mischler EH, Myers MG. Cephalexin compared to placebo in the management of patients with cystic fibrosis [abstract]. 19th Annual Meeting Cystic Fibrosis Club Abstracts. 1978:69.
-
- Loening‐Baucke V, Mischler EH, Myers MG. Cephalexin in cystic fibrosis: a placebo‐controlled study [abstract]. Pediatric Research 1978;12(4 Pt 2):495.
Nolan 1982 {published data only}
-
- Nolan G, McIvor P, Levinson H, Fleming PC, Corey M, Gold R. Antibiotic prophylaxis in cystic fibrosis: inhaled cephaloridine as an adjunct to oral cloxacillin. Journal of Pediatrics 1982;101(4):626‐30. - PubMed
Shapera 1981 {published data only}
-
- Shapera RM, Warwick WJ, Matsen JM. Clindamycin therapy of staphylococcal pulmonary infections in patients with cystic fibrosis. Journal of Pediatrics 1981;99(4):647‐50. - PubMed
Szaff 1982 {published data only}
-
- Szaff M, Hoiby N. Antibiotic treatment of staphylococcus aureus infection in cystic fibrosis. Acta Paediatrica Scandinavica 1982;71(5):821‐6. - PubMed
Wright 1970 {published data only}
-
- Wright GL, Harper J. Fusidic acid and lincomycin therapy in staphylococcal infections in cystic fibrosis. Lancet 1970;1(7636):9‐14. - PubMed
References to ongoing studies
CF START 2016 {published data only}
-
- 2016‐002578‐11. CF START [The cystic fibrosis (CF) anti‐staphylococcal antibiotic prophylaxis trial (CF START); a randomised registry trial to assess the safety and efficacy of flucloxacillin as a long‐term prophylaxis agent.]. www.clinicaltrialsregister.eu/ctr‐search/search?query=2016‐002578‐11 2016.
Additional references
Armstrong 1995
Armstrong 1996
-
- Armstrong DS, Grimwood K, Carlin JB, Carzino R, Olinsky A, Phelan PD. Bronchoalveolar lavage or oropharyngeal cultures to identify lower respiratory pathogens in infants with cystic fibrosis. Pediatric Pulmonology 1996;21(5):267‐75. - PubMed
Beardsmore 1994
Chrispin 1974
-
- Chrispin AR, Norman AP. The systematic evaluation of the chest radiograph in cystic fibrosis. Pediatric Radiology 1974;2:101‐5. - PubMed
Higgins 2011
-
- Higggins JPT, Altman DG, on behalf of the Cochrane Statistical Methods Group and the Cochrane Bias Methods Group. Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook of Systematic Reviews of Interventions. Version 5.1 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Kerem 1992
-
- Kerem E, Reisman J, Corey M, Canny GJ, Levison H. Prediction of mortality in patients with cystic fibrosis. New England Journal of Medicine 1992;326(18):1187‐91. - PubMed
Knudson 1983
-
- Knudson RJ, Lebowitz MD, Holberg CJ, Burrows B. Changes in the normal maximal expiratory flow‐volume curve with growth and aging. American Review of Respiratory Disease 1983;127(6):725–34. - PubMed
McCaffery 1999
Moher 2001
-
- Moher D, Schulz KF, Altman DG, for the CONSORT Group. The CONSORT statement: revised recommendations for improving the quality of reports of parallel group randomised trials. Lancet 2001;357(9263):1191‐4. - PubMed
Morison 1997
Pillarisetti 2011
-
- Pillarisetti N, Williamson E, Linnane B, Skoric B, Robertson CF, Robinson P, Massie J, Hall GL, Sly P, Stick S, Ranganathan S. Infection, inflammation, and lung function decline in infants with cystic fibrosis. American Journal of Respiratory & Critical Care Medicine 2011;184(1):75‐81. - PubMed
Shwachman 1958
-
- Shwachman H, Kulczycki LL. Long term study of one hundred five patients with cystic fibrosis. American Journal of Diseases of Children 1958;96:6‐15. - PubMed
References to other published versions of this review
Smyth 2003
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

