The pathway intermediate 2-keto-3-deoxy-L-galactonate mediates the induction of genes involved in D-galacturonic acid utilization in Aspergillus niger
- PMID: 28417461
- PMCID: PMC5488244
- DOI: 10.1002/1873-3468.12654
The pathway intermediate 2-keto-3-deoxy-L-galactonate mediates the induction of genes involved in D-galacturonic acid utilization in Aspergillus niger
Abstract
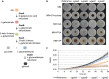
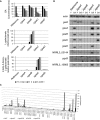
In Aspergillus niger, the enzymes encoded by gaaA, gaaB, and gaaC catabolize d-galacturonic acid (GA) consecutively into l-galactonate, 2-keto-3-deoxy-l-galactonate, pyruvate, and l-glyceraldehyde, while GaaD converts l-glyceraldehyde to glycerol. Deletion of gaaB or gaaC results in severely impaired growth on GA and accumulation of l-galactonate and 2-keto-3-deoxy-l-galactonate, respectively. Expression levels of GA-responsive genes are specifically elevated in the ∆gaaC mutant on GA as compared to the reference strain and other GA catabolic pathway deletion mutants. This indicates that 2-keto-3-deoxy-l-galactonate is the inducer of genes required for GA utilization.
Keywords: d-galacturonic acid catabolism; gene regulation; pectinase.
© 2017 The Authors. FEBS Letters published by John Wiley & Sons Ltd on behalf of Federation of European Biochemical Societies.
Figures
References
-
- Mohnen D (2008) Pectin structure and biosynthesis. Curr Opin Plant Biol 11, 266–277. - PubMed
-
- Martens‐Uzunova ES and Schaap PJ (2009) Assessment of the pectin degrading enzyme network of Aspergillus niger by functional genomics. Fungal Genet Biol 46, S170–S179. - PubMed
-
- Coutinho PM, Andersen MR, Kolenova K, Vankuyk PA, Benoit I, Gruben BS, Trejo‐Aguilar B, Visser H, van Solingen P, Pakula T et al (2009) Post‐genomic insights into the plant polysaccharide degradation potential of Aspergillus nidulans and comparison to Aspergillus niger and Aspergillus oryzae . Fungal Genet Biol 46, S161–S169. - PubMed
-
- Martens‐Uzunova ES and Schaap PJ (2008) An evolutionary conserved D‐galacturonic acid metabolic pathway operates across filamentous fungi capable of pectin degradation. Fungal Genet Biol 45, 1449–1457. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

