Eluxadoline Efficacy in IBS-D Patients Who Report Prior Loperamide Use
- PMID: 28417992
- PMCID: PMC5465428
- DOI: 10.1038/ajg.2017.72
Eluxadoline Efficacy in IBS-D Patients Who Report Prior Loperamide Use
Erratum in
-
Corrigendum: Eluxadoline Efficacy in IBS-D Patients Who Report Prior Loperamide Use.Am J Gastroenterol. 2017 Jul;112(7):1210. doi: 10.1038/ajg.2017.152. Epub 2017 May 30. Am J Gastroenterol. 2017. PMID: 28555632
Abstract
Objectives: Irritable bowel syndrome with diarrhea (IBS-D) is often managed with over-the-counter therapies such as loperamide, though with limited success. This analysis evaluated the efficacy of eluxadoline in patients previously treated with loperamide in two phase 3 studies.
Methods: Adults with IBS-D (Rome III criteria) were enrolled and randomized to placebo or eluxadoline (75 or 100 mg) twice daily for 26 (IBS-3002) or 52 (IBS-3001) weeks. Patients reported loperamide use over the previous year and recorded their rescue loperamide use during the studies. The primary efficacy end point was the proportion of patients with a composite response of simultaneous improvement in abdominal pain and reduction in diarrhea.
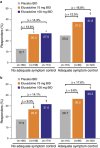
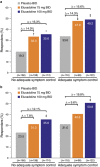
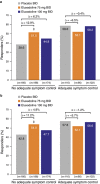
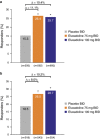
Results: A total of 2,428 patients were enrolled; 36.0% reported prior loperamide use, of whom 61.8% reported prior inadequate IBS-D symptom control with loperamide. Among patients with prior loperamide use, a greater proportion treated with eluxadoline (75 and 100 mg) were composite responders vs. those treated with placebo with inadequate prior symptom control, over weeks 1-12 (26.3% (P=0.001) and 27.0% (P<0.001) vs. 12.7%, respectively); similar results were observed over weeks 1-26. When daily rescue loperamide use was imputed as a nonresponse day, the composite responder rate was still higher in patients receiving eluxadoline (75 and 100 mg) vs. placebo over weeks 1-12 (P<0.001) and weeks 1-26 (P<0.001). Adverse events included nausea and abdominal pain.
Conclusions: Eluxadoline effectively and safely treats IBS-D symptoms of abdominal pain and diarrhea in patients who self-report either adequate or inadequate control of their symptoms with prior loperamide treatment, with comparable efficacy and safety irrespective of the use of loperamide as a rescue medication during eluxadoline treatment.
Conflict of interest statement
Figures

Similar articles
-
Efficacy and Safety of Eluxadoline in Patients With Irritable Bowel Syndrome With Diarrhea Who Report Inadequate Symptom Control With Loperamide: RELIEF Phase 4 Study.Am J Gastroenterol. 2019 Sep;114(9):1502-1511. doi: 10.14309/ajg.0000000000000327. Am J Gastroenterol. 2019. PMID: 31356229 Free PMC article. Clinical Trial.
-
Improved work productivity and health-related quality of life in patients with irritable bowel syndrome with diarrhea receiving eluxadoline following inadequate response to loperamide.J Manag Care Spec Pharm. 2021 Apr;27(4):469-477. doi: 10.18553/jmcp.2021.27.4.469. J Manag Care Spec Pharm. 2021. PMID: 33769858 Free PMC article.
-
Eluxadoline benefits patients with irritable bowel syndrome with diarrhea in a phase 2 study.Gastroenterology. 2013 Aug;145(2):329-38.e1. doi: 10.1053/j.gastro.2013.04.006. Epub 2013 Apr 9. Gastroenterology. 2013. PMID: 23583433 Clinical Trial.
-
Update on the Management of Diarrhea-Predominant Irritable Bowel Syndrome: Focus on Rifaximin and Eluxadoline.Pharmacotherapy. 2016 Mar;36(3):300-16. doi: 10.1002/phar.1712. Epub 2016 Mar 11. Pharmacotherapy. 2016. PMID: 26971716 Review.
-
Update on Eluxadoline for the Treatment of Irritable Bowel Syndrome with Diarrhea: Patient Selection and Perspectives.Drug Des Devel Ther. 2020 Apr 9;14:1391-1400. doi: 10.2147/DDDT.S216056. eCollection 2020. Drug Des Devel Ther. 2020. PMID: 32308371 Free PMC article. Review.
Cited by
-
Evaluation of the effectiveness and mechanism of action of the Chang-Kang-Fang formula combined with bifid triple viable capsules on diarrhea-predominant irritable bowel syndrome.Front Microbiol. 2023 Jun 27;14:1160783. doi: 10.3389/fmicb.2023.1160783. eCollection 2023. Front Microbiol. 2023. PMID: 37440881 Free PMC article.
-
Targeting mu opioid receptors to modulate gastrointestinal function: what have we learnt so far from the studies in functional bowel disorders?F1000Res. 2019 Mar 5;8:F1000 Faculty Rev-257. doi: 10.12688/f1000research.15974.1. eCollection 2019. F1000Res. 2019. PMID: 30863534 Free PMC article. Review.
-
The place of eluxadoline in the management of irritable bowel syndrome with diarrhea.Therap Adv Gastroenterol. 2017 Sep;10(9):715-725. doi: 10.1177/1756283X17721152. Epub 2017 Jul 24. Therap Adv Gastroenterol. 2017. PMID: 28932272 Free PMC article. Review.
-
Understanding and Managing IBS and CIC in the Primary Care Setting.Gastroenterol Hepatol (N Y). 2018 May;14(5 Suppl 3):3-15. Gastroenterol Hepatol (N Y). 2018. PMID: 30279636 Free PMC article. No abstract available.
-
Gut Movements: A Review of the Physiology of Gastrointestinal Transit.Dig Dis Sci. 2018 Oct;63(10):2500-2506. doi: 10.1007/s10620-018-5259-1. Dig Dis Sci. 2018. PMID: 30145693 Review.
References
-
- Longstreth GF, Thompson WG, Chey WD et al. Functional bowel disorders. Gastroenterology 2006;130:1480–1491. - PubMed
-
- Chey WD, Kurlander J, Eswaran S. Irritable bowel syndrome: a clinical review. JAMA 2015;313:949–958. - PubMed
-
- Lovell RM, Ford AC. Global prevalence of and risk factors for irritable bowel syndrome: a meta-analysis. Clin Gastroenterol Hepatol 2012;10:712–721. - PubMed
-
- Gralnek IM, Hays RD, Kilbourne A et al. The impact of irritable bowel syndrome on health-related quality of life. Gastroenterology 2000;119:654–660. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

