Iterative expansion microscopy
- PMID: 28417997
- PMCID: PMC5560071
- DOI: 10.1038/nmeth.4261
Iterative expansion microscopy
Abstract
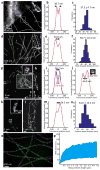
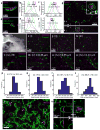
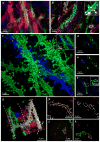
We recently developed a method called expansion microscopy, in which preserved biological specimens are physically magnified by embedding them in a densely crosslinked polyelectrolyte gel, anchoring key labels or biomolecules to the gel, mechanically homogenizing the specimen, and then swelling the gel-specimen composite by ∼4.5× in linear dimension. Here we describe iterative expansion microscopy (iExM), in which a sample is expanded ∼20×. After preliminary expansion a second swellable polymer mesh is formed in the space newly opened up by the first expansion, and the sample is expanded again. iExM expands biological specimens ∼4.5 × 4.5, or ∼20×, and enables ∼25-nm-resolution imaging of cells and tissues on conventional microscopes. We used iExM to visualize synaptic proteins, as well as the detailed architecture of dendritic spines, in mouse brain circuitry.
Conflict of interest statement
E.S.B., J.-B.C., F.C., and P.W.T. have applied for a patent on iExM. E.S.B. is co-founder of a company, Expansion Technologies, that aims to provide expansion microscopy kits and services to the community.
Figures
Similar articles
-
Magnify is a universal molecular anchoring strategy for expansion microscopy.Nat Biotechnol. 2023 Jun;41(6):858-869. doi: 10.1038/s41587-022-01546-1. Epub 2023 Jan 2. Nat Biotechnol. 2023. PMID: 36593399 Free PMC article.
-
Protein-retention expansion microscopy of cells and tissues labeled using standard fluorescent proteins and antibodies.Nat Biotechnol. 2016 Sep;34(9):987-92. doi: 10.1038/nbt.3625. Epub 2016 Jul 4. Nat Biotechnol. 2016. PMID: 27376584 Free PMC article.
-
Q&A: Expansion microscopy.BMC Biol. 2017 Jun 19;15(1):50. doi: 10.1186/s12915-017-0393-3. BMC Biol. 2017. PMID: 28629474 Free PMC article.
-
Expansion microscopy: development and neuroscience applications.Curr Opin Neurobiol. 2018 Jun;50:56-63. doi: 10.1016/j.conb.2017.12.012. Epub 2018 Jan 6. Curr Opin Neurobiol. 2018. PMID: 29316506 Free PMC article. Review.
-
Recent advances in electron imaging, image interpretation and applications: environmental scanning electron microscopy.Philos Trans A Math Phys Eng Sci. 2003 Dec 15;361(1813):2771-87. doi: 10.1098/rsta.2003.1279. Philos Trans A Math Phys Eng Sci. 2003. PMID: 14667297 Review.
Cited by
-
PICASSO allows ultra-multiplexed fluorescence imaging of spatially overlapping proteins without reference spectra measurements.Nat Commun. 2022 May 5;13(1):2475. doi: 10.1038/s41467-022-30168-z. Nat Commun. 2022. PMID: 35513404 Free PMC article.
-
Simultaneous expansion microscopy imaging of proteins and mRNAs via dual-ExM.Sci Rep. 2022 Mar 1;12(1):3360. doi: 10.1038/s41598-022-06903-3. Sci Rep. 2022. PMID: 35233025 Free PMC article.
-
Nanoscale fluorescence imaging of biological ultrastructure via molecular anchoring and physical expansion.Nano Converg. 2022 Jul 9;9(1):30. doi: 10.1186/s40580-022-00318-6. Nano Converg. 2022. PMID: 35810234 Free PMC article. Review.
-
Expansion Microscopy for Beginners: Visualizing Microtubules in Expanded Cultured HeLa Cells.Curr Protoc Neurosci. 2020 Jun;92(1):e96. doi: 10.1002/cpns.96. Curr Protoc Neurosci. 2020. PMID: 32497404 Free PMC article.
-
Proximity Labeling Expansion Microscopy (PL-ExM) resolves structure of the interactome.bioRxiv [Preprint]. 2023 Nov 13:2023.11.09.566477. doi: 10.1101/2023.11.09.566477. bioRxiv. 2023. Update in: J Mater Chem B. 2024 Aug 28;12(34):8335-8348. doi: 10.1039/d4tb00516c. PMID: 38014020 Free PMC article. Updated. Preprint.
References
-
- O’Connell PBH, Brady CJ. Polyacrylamide gels with modified cross-linkages. Anal Biochem. 1976;76:63–73. - PubMed
-
- Kurenkov VF, Hartan HG, Lobanov FI. Alkaline Hydrolysis of Polyacrylamide. Russ J Appl Chem. 2001;74:543–554.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

