5-methylcytosine promotes mRNA export - NSUN2 as the methyltransferase and ALYREF as an m5C reader
- PMID: 28418038
- PMCID: PMC5594206
- DOI: 10.1038/cr.2017.55
5-methylcytosine promotes mRNA export - NSUN2 as the methyltransferase and ALYREF as an m5C reader
Abstract
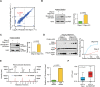
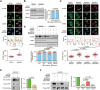
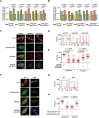
5-methylcytosine (m5C) is a post-transcriptional RNA modification identified in both stable and highly abundant tRNAs and rRNAs, and in mRNAs. However, its regulatory role in mRNA metabolism is still largely unknown. Here, we reveal that m5C modification is enriched in CG-rich regions and in regions immediately downstream of translation initiation sites and has conserved, tissue-specific and dynamic features across mammalian transcriptomes. Moreover, m5C formation in mRNAs is mainly catalyzed by the RNA methyltransferase NSUN2, and m5C is specifically recognized by the mRNA export adaptor ALYREF as shown by in vitro and in vivo studies. NSUN2 modulates ALYREF's nuclear-cytoplasmic shuttling, RNA-binding affinity and associated mRNA export. Dysregulation of ALYREF-mediated mRNA export upon NSUN2 depletion could be restored by reconstitution of wild-type but not methyltransferase-defective NSUN2. Our study provides comprehensive m5C profiles of mammalian transcriptomes and suggests an essential role for m5C modification in mRNA export and post-transcriptional regulation.
Figures





References
-
- Dominissini D, Moshitch-Moshkovitz S, Schwartz S, et al. Topology of the human and mouse m6A RNA methylomes revealed by m6A-seq. Nature 2012; 485:201–206. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

