Water-Ice Analogues of Polycyclic Aromatic Hydrocarbons: Water Nanoclusters on Cu(111)
- PMID: 28418246
- PMCID: PMC5432957
- DOI: 10.1021/jacs.7b01883
Water-Ice Analogues of Polycyclic Aromatic Hydrocarbons: Water Nanoclusters on Cu(111)
Abstract
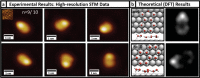
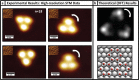
Water has an incredible ability to form a rich variety of structures, with 16 bulk ice phases identified, for example, as well as numerous distinct structures for water at interfaces or under confinement. Many of these structures are built from hexagonal motifs of water molecules, and indeed, for water on metal surfaces, individual hexamers of just six water molecules have been observed. Here, we report the results of low-temperature scanning tunneling microscopy experiments and density functional theory calculations which reveal a host of new structures for water-ice nanoclusters when adsorbed on an atomically flat Cu surface. The H-bonding networks within the nanoclusters resemble the resonance structures of polycyclic aromatic hydrocarbons, and water-ice analogues of inene, naphthalene, phenalene, anthracene, phenanthrene, and triphenylene have been observed. The specific structures identified and the H-bonding patterns within them reveal new insight about water on metals that allows us to refine the so-called "2D ice rules", which have so far proved useful in understanding water-ice structures at solid surfaces.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

