BTKC481S-Mediated Resistance to Ibrutinib in Chronic Lymphocytic Leukemia
- PMID: 28418267
- PMCID: PMC5455463
- DOI: 10.1200/JCO.2016.70.2282
BTKC481S-Mediated Resistance to Ibrutinib in Chronic Lymphocytic Leukemia
Abstract
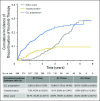
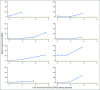
Purpose Therapeutic targeting of Bruton tyrosine kinase (BTK) with ibrutinib in chronic lymphocytic leukemia has led to a paradigm shift in therapy, and relapse has been uncommon with current follow-up. Acquired mutations in BTK and PLCG2 can cause relapse, but data regarding the prevalence and natural history of these mutations are limited. Patients and Methods Patients accrued to four sequential studies of ibrutinib were included in these analyses. Deep sequencing for BTK and PLCG2 was performed retrospectively on patients who experienced relapse and prospectively on a screening population. Results With a median follow-up time of 3.4 years, the estimated cumulative incidence of progression at 4 years is 19% (95% CI, 14% to 24%). Baseline karyotypic complexity, presence of del(17)(p13.1), and age less than 65 years were risk factors for progression. Among patients who experienced relapse, acquired mutations of BTK or PLCG2 were found in 85% (95% CI, 71% to 94%), and these mutations were detected an estimated median of 9.3 months (95% CI, 7.6 to 11.7 months) before relapse. Of a group of 112 patients examined prospectively, eight patients have experienced relapse, and all of these patients had acquired resistance mutations before relapse. A resistance mutation was detected in an additional eight patients who have not yet met criteria for clinical relapse. Conclusion Relapse of chronic lymphocytic leukemia after ibrutinib is an issue of increasing clinical significance. We show that mutations in BTK and PLCG2 appear early and have the potential to be used as a biomarker for future relapse, suggesting an opportunity for intervention.
Figures


References
-
- Fowler N, Sharman JP, Smith SM, et al: The Btk inhibitor, PCI-32765, induces durable responses with minimal toxicity in patients with relapsed/refractory B-cell malignancies: Results from a phase I study. Blood 116:964, 2010 (abstr)
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

