Sequential sensory and decision processing in posterior parietal cortex
- PMID: 28418332
- PMCID: PMC5422072
- DOI: 10.7554/eLife.23743
Sequential sensory and decision processing in posterior parietal cortex
Abstract
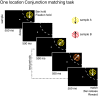
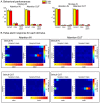
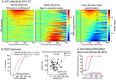
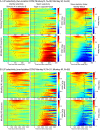
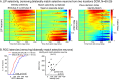
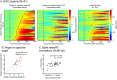
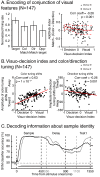
Decisions about the behavioral significance of sensory stimuli often require comparing sensory inference of what we are looking at to internal models of what we are looking for. Here, we test how neuronal selectivity for visual features is transformed into decision-related signals in posterior parietal cortex (area LIP). Monkeys performed a visual matching task that required them to detect target stimuli composed of conjunctions of color and motion-direction. Neuronal recordings from area LIP revealed two main findings. First, the sequential processing of visual features and the selection of target-stimuli suggest that LIP is involved in transforming sensory information into decision-related signals. Second, the patterns of color and motion selectivity and their impact on decision-related encoding suggest that LIP plays a role in detecting target stimuli by comparing bottom-up sensory inputs (what the monkeys were looking at) and top-down cognitive encoding inputs (what the monkeys were looking for).
Keywords: Parietal Cortex; Prefrontal Cortex; attention; decision making; neuroscience; rhesus macaque; vision; visual perception.
Conflict of interest statement
The authors declare that no competing interests exist.
Figures




References
-
- Barash S, Bracewell RM, Fogassi L, Gnadt JW, Andersen RA. Saccade-related activity in the lateral intraparietal area. I. temporal properties; comparison with area 7a. Journal of Neurophysiology. 1991;66:1095–1108. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

