A Liposome Encapsulated Ruthenium Polypyridine Complex as a Theranostic Platform for Triple-Negative Breast Cancer
- PMID: 28418672
- PMCID: PMC5484597
- DOI: 10.1021/acs.nanolett.7b00132
A Liposome Encapsulated Ruthenium Polypyridine Complex as a Theranostic Platform for Triple-Negative Breast Cancer
Abstract
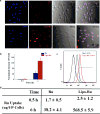
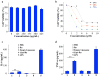
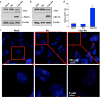
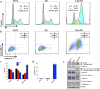
Ruthenium coordination complexes have the potential to serve as novel theranostic agents for cancer. However, a major limitation in their clinical implementation is effective tumor accumulation. In this study, we have developed a liposome-based theranostic nanodelivery system for [Ru(phen)2dppz](ClO4)2 (Lipo-Ru). This ruthenium polypyridine complex emits a strong fluorescent signal when incorporated in the hydrophobic lipid bilayer of the delivery vehicle or in the DNA helix, enabling visualization of the therapeutic agent in tumor tissues. Incubation of MDA-MB-231 breast cancer cells with Lipo-Ru induced double-strand DNA breaks and triggers apoptosis. In a mouse model of triple-negative breast cancer, treatment with Lipo-Ru dramatically reduced tumor growth. Biodistribution studies of Lipo-Ru revealed that more than 20% of the injected dose accumulated in the tumor. These results suggest that Lipo-Ru could serve as a promising theranostic platform for cancer.
Keywords: Liposome; nanoplatform; ruthenium polypyridine complex; theranostic; triple-negative breast cancer.
Conflict of interest statement
Notes
The authors declare no competing financial interest.
Figures

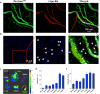
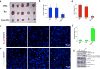

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

