A Two-Biomarker Model Predicts Mortality in the Critically Ill with Sepsis
- PMID: 28418697
- PMCID: PMC5649981
- DOI: 10.1164/rccm.201611-2307OC
A Two-Biomarker Model Predicts Mortality in the Critically Ill with Sepsis
Abstract
Rationale: Improving the prospective identification of patients with systemic inflammatory response syndrome (SIRS) and sepsis at low risk for organ dysfunction and death is a major clinical challenge.
Objectives: To develop and validate a multibiomarker-based prediction model for 28-day mortality in critically ill patients with SIRS and sepsis.
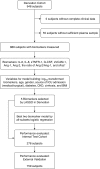
Methods: A derivation cohort (n = 888) and internal test cohort (n = 278) were taken from a prospective study of critically ill intensive care unit (ICU) patients meeting two of four SIRS criteria at an academic medical center for whom plasma was obtained within 24 hours. The validation cohort (n = 759) was taken from a prospective cohort enrolled at another academic medical center ICU for whom plasma was obtained within 48 hours. We measured concentrations of angiopoietin-1, angiopoietin-2, IL-6, IL-8, soluble tumor necrosis factor receptor-1, soluble vascular cell adhesion molecule-1, granulocyte colony-stimulating factor, and soluble Fas.
Measurements and main results: We identified a two-biomarker model in the derivation cohort that predicted mortality (area under the receiver operator characteristic curve [AUC], 0.79; 95% confidence interval [CI], 0.74-0.83). It performed well in the internal test cohort (AUC, 0.75; 95% CI, 0.65-0.85) and the external validation cohort (AUC, 0.77; 95% CI, 0.72-0.83). We determined a model score threshold demonstrating high negative predictive value (0.95) for death. In addition to a low risk of death, patients below this threshold had shorter ICU length of stay, lower incidence of acute kidney injury, acute respiratory distress syndrome, and need for vasopressors.
Conclusions: We have developed a simple, robust biomarker-based model that identifies patients with SIRS/sepsis at low risk for death and organ dysfunction.
Keywords: IL-8; biomarkers; sepsis; systemic inflammatory response syndrome; tumor necrosis factor receptor.
Figures

Comment in
-
Biomarkers in Critical Illness: New Insights and Challenges for the Future.Am J Respir Crit Care Med. 2017 Oct 15;196(8):944-945. doi: 10.1164/rccm.201704-0831ED. Am J Respir Crit Care Med. 2017. PMID: 28475361 No abstract available.
References
-
- Gaieski DF, Edwards JM, Kallan MJ, Carr BG. Benchmarking the incidence and mortality of severe sepsis in the United States. Crit Care Med. 2013;41:1167–1174. - PubMed
-
- Stoller J, Halpin L, Weis M, Aplin B, Qu W, Georgescu C, Nazzal M. Epidemiology of severe sepsis: 2008-2012. J Crit Care. 2016;31:58–62. - PubMed
-
- Vincent J-L, Marshall JC, Namendys-Silva SA, François B, Martin-Loeches I, Lipman J, Reinhart K, Antonelli M, Pickkers P, Njimi H, et al. ICON investigators. Assessment of the worldwide burden of critical illness: the Intensive Care Over Nations (ICON) audit. Lancet Respir Med. 2014;2:380–386. - PubMed
-
- Fleischmann C, Scherag A, Adhikari NKJ, Hartog CS, Tsaganos T, Schlattmann P, Angus DC, Reinhart K International Forum of Acute Care Trialists. International Forum of Acute Care Trialists. Assessment of global incidence and mortality of hospital-treated sepsis. current estimates and limitations. Am J Respir Crit Care Med. 2016;193:259–272. - PubMed
-
- Vincent J-L, Rello J, Marshall J, Silva E, Anzueto A, Martin CD, Moreno R, Lipman J, Gomersall C, Sakr Y, et al. EPIC II Group of Investigators. EPIC II Group of Investigators. International study of the prevalence and outcomes of infection in intensive care units. JAMA. 2009;302:2323–2329. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

