OSU-A9 inhibits pancreatic cancer cell lines by modulating p38-JAK-STAT3 signaling
- PMID: 28418923
- PMCID: PMC5438726
- DOI: 10.18632/oncotarget.16450
OSU-A9 inhibits pancreatic cancer cell lines by modulating p38-JAK-STAT3 signaling
Abstract
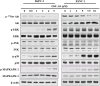
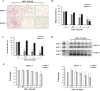
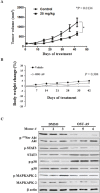
Pancreatic cancer is an aggressive malignancy that is the fourth leading cause of death worldwide. Since there is a dire need for novel and effective therapies to improve the poor survival rates of advanced pancreatic cancer patients, we analyzed the antitumor effects of OSU-A9, an indole-3-carbinol derivative, on pancreatic cancer cell lines in vitro and in vivo. OSU-A9 exhibited a stronger antitumor effect than gemcitabine on two pancreatic cancer cell lines, including gemcitabine-resistant PANC-1 cells. OSU-A9 treatment induced apoptosis, the down-regulation of Akt phosphorylation, up-regulation of p38 phosphorylation and decreased phosphorylation of JAK and STAT3. Cell migration and invasiveness assays showed that OSU-A9 reduced cancer cell aggressiveness and inhibited BxPC-3 xenograft growth in nude mice. These results suggest that OSU-A9 modulates the p38-JAK-STAT3 signaling module, thereby inducing cytotoxicity in pancreatic cancer cells. Continued evaluation of OSU-A9 as a potential therapeutic agent for pancreatic cancer thus appears warrented.
Keywords: JAK; OSU-A9; STAT3; p38; pancreatic cancer.
Conflict of interest statement
The authors declare no competing financial interests.
Figures



Similar articles
-
OSU-A9, an indole-3-carbinol derivative, induces cytotoxicity in acute myeloid leukemia through reactive oxygen species-mediated apoptosis.Biochem Pharmacol. 2013 Nov 15;86(10):1430-40. doi: 10.1016/j.bcp.2013.09.002. Epub 2013 Sep 13. Biochem Pharmacol. 2013. PMID: 24041743
-
A potent indole-3-carbinol derived antitumor agent with pleiotropic effects on multiple signaling pathways in prostate cancer cells.Cancer Res. 2007 Aug 15;67(16):7815-24. doi: 10.1158/0008-5472.CAN-07-0794. Cancer Res. 2007. PMID: 17699787
-
A novel indole-3-carbinol derivative inhibits the growth of human oral squamous cell carcinoma in vitro.Oral Oncol. 2010 Oct;46(10):748-54. doi: 10.1016/j.oraloncology.2010.08.005. Epub 2010 Sep 16. Oral Oncol. 2010. PMID: 20843730
-
Targeting of the Akt-nuclear factor-kappa B signaling network by [1-(4-chloro-3-nitrobenzenesulfonyl)-1H-indol-3-yl]-methanol (OSU-A9), a novel indole-3-carbinol derivative, in a mouse model of hepatocellular carcinoma.Mol Pharmacol. 2009 Nov;76(5):957-68. doi: 10.1124/mol.109.058180. Epub 2009 Aug 25. Mol Pharmacol. 2009. PMID: 19706731 Free PMC article.
-
OSU-A9, a potent indole-3-carbinol derivative, suppresses breast tumor growth by targeting the Akt-NF-kappaB pathway and stress response signaling.Carcinogenesis. 2009 Oct;30(10):1702-9. doi: 10.1093/carcin/bgp202. Epub 2009 Aug 25. Carcinogenesis. 2009. PMID: 19706645 Free PMC article.
Cited by
-
microRNA-1225 inhibit apoptosis of pancreatic cancer cells via targeting JAK1.Cell Cycle. 2019 May;18(9):990-1000. doi: 10.1080/15384101.2019.1608127. Epub 2019 Apr 24. Cell Cycle. 2019. PMID: 30990343 Free PMC article.
-
Kinome-Wide siRNA Screening Identifies Src-Enhanced Resistance of Chemotherapeutic Drugs in Triple-Negative Breast Cancer Cells.Front Pharmacol. 2018 Nov 9;9:1285. doi: 10.3389/fphar.2018.01285. eCollection 2018. Front Pharmacol. 2018. PMID: 30473665 Free PMC article.
-
TR35 Exerts Anti-tumor Effects by Modulating Mitogen-Activated Protein Kinase and STAT3 Signaling in Lung Cancer Cells.Front Cell Dev Biol. 2021 Oct 25;9:723346. doi: 10.3389/fcell.2021.723346. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34760885 Free PMC article.
-
The anti-dysenteric drug fraxetin enhances anti-tumor efficacy of gemcitabine and suppresses pancreatic cancer development by antagonizing STAT3 activation.Aging (Albany NY). 2021 Jul 28;13(14):18545-18563. doi: 10.18632/aging.203301. Epub 2021 Jul 28. Aging (Albany NY). 2021. PMID: 34320467 Free PMC article.
References
-
- Weng JR, Omar HA, Kulp SK, Chen CS. Pharmacological exploitation of indole-3-carbinol to develop potent antitumor agents. Mini Rev Med Chem. 2010;10:398–404. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

