Effectiveness of a live oral human rotavirus vaccine after programmatic introduction in Bangladesh: A cluster-randomized trial
- PMID: 28419095
- PMCID: PMC5395158
- DOI: 10.1371/journal.pmed.1002282
Effectiveness of a live oral human rotavirus vaccine after programmatic introduction in Bangladesh: A cluster-randomized trial
Abstract
Background: Rotavirus vaccines are now globally recommended by the World Health Organization (WHO), but in early 2009 WHO's Strategic Advisory Group of Experts on Immunization reviewed available data and concluded that there was no evidence for the efficacy or effectiveness of a two-dose schedule of the human rotavirus vaccine (HRV; Rotarix) given early at 6 and 10 wk of age. Additionally, the effectiveness of programmatic rotavirus vaccination, including possible indirect effects, has not been assessed in low-resource populations in Asia.
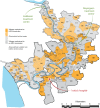
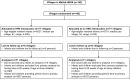
Methods and findings: In Bangladesh, we cluster-randomized (1:1) 142 villages of the Matlab Health and Demographic Surveillance System to include two doses of HRV with the standard infant vaccines at 6 and 10 wk of age or to provide standard infant vaccines without HRV. The study was initiated November 1, 2008, and surveillance was conducted concurrently at Matlab Diarrhoea Hospital and two community treatment centers to identify children less than 2 y of age presenting with acute rotavirus diarrhea (ARD) through March 31, 2011. Laboratory confirmation was made by enzyme immunoassay detection of rotavirus antigen in stool specimens. Overall effectiveness of the HRV vaccination program (primary objective) was measured by comparing the incidence rate of ARD among all children age-eligible for vaccination in villages where HRV was introduced to that among such children in villages where HRV was not introduced. Total effectiveness among vaccinees and indirect effectiveness were also evaluated. In all, 6,527 infants were age-eligible for vaccination in 71 HRV villages, and 5,791 in 71 non-HRV villages. In HRV villages, 4,808 (73.7%) infants received at least one dose of HRV. The incidence rate of ARD was 4.10 cases per 100 person-years in non-HRV villages compared to 2.8 per 100 person-years in HRV villages, indicating an overall effectiveness of 29.0% (95% CI, 11.3% to 43.1%). The total effectiveness of HRV against ARD among vaccinees was 41.4% (95% CI, 23.2% to 55.2%). The point estimate for total effectiveness was higher against ARD during the first year of life than during the second (45.2% versus 28.9%), but estimates for the second year of life lacked precision and did not reach statistical significance. Indirect effects were not detected. To check for bias in presentation to treatment facilities, we evaluated the effectiveness of HRV against acute diarrhea associated with enterotoxigenic Escherichia coli; it was 4.0% (95% CI, -46.5% to 37.1%), indicating that bias likely was not introduced. Thirteen serious adverse events were identified among recipients of HRV, but none were considered related to receipt of study vaccine. The main limitation of this study is that it was an open-label study with an observed-only control group (no placebo).
Conclusions: The two-dose HRV rotavirus vaccination program significantly reduced medically attended ARD in this low-resource population in Asia. Protection among vaccinees was similar to that in other low-resource settings. In low-resource populations with high rotavirus incidence, large-scale vaccination across a wide population may be required to obtain the full benefit of rotavirus vaccination, including indirect effects.
Trial registration: ClinicalTrials.gov NCT00737503.
Conflict of interest statement
I have read the journal's policy and the authors of this manuscript have the following competing interests: During this research, KNM, JAF, KDCL and JCV's employment at PATH was supported by research grants for rotavirus vaccines from the Gavi Alliance and the Bill & Melinda Gates Foundation. During the writing of this manuscript, KDCL was an employee of the Bill & Melinda Gates Foundation on the Pneumonia team. During the writing of this manuscript, AJF was employed by Novartis Vaccines when it was acquired by GlaxoSmithKline, the maker of Rotarix; however, for the six-month period while AJF was employed by GSK, she did not work on company projects related to rotavirus vaccines. LHM serves on an advisory board for Merck for one of its respiratory vaccines; Merck has a competitor rotavirus vaccine.
Figures
Comment in
-
Rotavirus vaccine will have an impact in Asia.PLoS Med. 2017 May 9;14(5):e1002298. doi: 10.1371/journal.pmed.1002298. eCollection 2017 May. PLoS Med. 2017. PMID: 28486524 Free PMC article.
References
-
- Kotloff KL, Nataro JP, Blackwelder WC, Nasrin D, Farag TH, Panchalingam S, et al. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): a prospective, case-control study. Lancet. 2016;382:209–22. - PubMed
-
- Conclusions and recommendations from the Immunization Strategic Advisory Group. Wkly Epidemiol Rec. 2006;81:2–11. - PubMed
-
- Armah GE, Sow SO, Breiman RF, Dallas MJ, Tapia MD, Feikin DR, et al. Efficacy of pentavalent rotavirus vaccine against severe rotavirus gastroenteritis in infants in developing countries in sub-Saharan Africa: a randomised, double-blind, placebo-controlled trial. Lancet. 2016;376:606–14. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

