Cytoskeletal stability and metabolic alterations in primary human macrophages in long-term microgravity
- PMID: 28419128
- PMCID: PMC5395169
- DOI: 10.1371/journal.pone.0175599
Cytoskeletal stability and metabolic alterations in primary human macrophages in long-term microgravity
Abstract
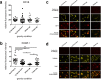
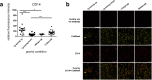
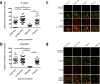
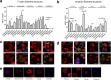
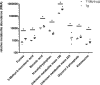
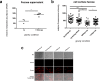
The immune system is one of the most affected systems of the human body during space flight. The cells of the immune system are exceptionally sensitive to microgravity. Thus, serious concerns arise, whether space flight associated weakening of the immune system ultimately precludes the expansion of human presence beyond the Earth's orbit. For human space flight, it is an urgent need to understand the cellular and molecular mechanisms by which altered gravity influences and changes the functions of immune cells. The CELLBOX-PRIME (= CellBox-Primary Human Macrophages in Microgravity Environment) experiment investigated for the first time microgravity-associated long-term alterations in primary human macrophages, one of the most important effector cells of the immune system. The experiment was conducted in the U.S. National Laboratory on board of the International Space Station ISS using the NanoRacks laboratory and Biorack type I standard CELLBOX EUE type IV containers. Upload and download were performed with the SpaceX CRS-3 and the Dragon spaceship on April 18th, 2014 / May 18th, 2014. Surprisingly, primary human macrophages exhibited neither quantitative nor structural changes of the actin and vimentin cytoskeleton after 11 days in microgravity when compared to 1g controls. Neither CD18 or CD14 surface expression were altered in microgravity, however ICAM-1 expression was reduced. The analysis of 74 metabolites in the cell culture supernatant by GC-TOF-MS, revealed eight metabolites with significantly different quantities when compared to 1g controls. In particular, the significant increase of free fucose in the cell culture supernatant was associated with a significant decrease of cell surface-bound fucose. The reduced ICAM-1 expression and the loss of cell surface-bound fucose may contribute to functional impairments, e.g. the activation of T cells, migration and activation of the innate immune response. We assume that the surprisingly small and non-significant cytoskeletal alterations represent a stable "steady state" after adaptive processes are initiated in the new microgravity environment. Due to the utmost importance of the human macrophage system for the elimination of pathogens and the clearance of apoptotic cells, its apparent robustness to a low gravity environment is crucial for human health and performance during long-term space missions.
Conflict of interest statement
Figures




Similar articles
-
Rapid Morphological and Cytoskeletal Response to Microgravity in Human Primary Macrophages.Int J Mol Sci. 2019 May 15;20(10):2402. doi: 10.3390/ijms20102402. Int J Mol Sci. 2019. PMID: 31096581 Free PMC article.
-
Regulation of ICAM-1 in cells of the monocyte/macrophage system in microgravity.Biomed Res Int. 2015;2015:538786. doi: 10.1155/2015/538786. Epub 2015 Jan 13. Biomed Res Int. 2015. PMID: 25654110 Free PMC article.
-
The microgravity environment for experiments on the International Space Station.J Gravit Physiol. 2004 Mar;11(1):1-10. J Gravit Physiol. 2004. PMID: 16145793
-
Function of the cytoskeleton in gravisensing during spaceflight.Adv Space Res. 2003;32(8):1585-93. doi: 10.1016/S0273-1177(03)90399-1. Adv Space Res. 2003. PMID: 15002415 Review.
-
Microgravity: the immune response and bone.Immunol Rev. 2005 Dec;208:267-80. doi: 10.1111/j.0105-2896.2005.00330.x. Immunol Rev. 2005. PMID: 16313354 Review.
Cited by
-
Short-Term Microgravity Influences Cell Adhesion in Human Breast Cancer Cells.Int J Mol Sci. 2019 Nov 15;20(22):5730. doi: 10.3390/ijms20225730. Int J Mol Sci. 2019. PMID: 31731625 Free PMC article.
-
A Protective Strategy to Counteract the Oxidative Stress Induced by Simulated Microgravity on H9C2 Cardiomyocytes.Oxid Med Cell Longev. 2021 Apr 20;2021:9951113. doi: 10.1155/2021/9951113. eCollection 2021. Oxid Med Cell Longev. 2021. PMID: 33986919 Free PMC article.
-
In Vitro Models of Bone Marrow Remodelling and Immune Dysfunction in Space: Present State and Future Directions.Biomedicines. 2022 Mar 24;10(4):766. doi: 10.3390/biomedicines10040766. Biomedicines. 2022. PMID: 35453515 Free PMC article. Review.
-
May the Force Be with You (Or Not): The Immune System under Microgravity.Cells. 2021 Jul 30;10(8):1941. doi: 10.3390/cells10081941. Cells. 2021. PMID: 34440709 Free PMC article. Review.
-
Longitudinal metabolomic profiles reveal sex-specific adjustments to long-duration spaceflight and return to Earth.Cell Mol Life Sci. 2022 Nov 1;79(11):578. doi: 10.1007/s00018-022-04566-x. Cell Mol Life Sci. 2022. PMID: 36319708 Free PMC article.
References
-
- Comet B. Limiting factors for human health and performance: microgravity and re-duced gravity. In: Study on the survivability and adaptation of humans to long-duration interplanetary and planetary environments; Technical Note 2: Critical assessments of the limiting factors for human health and performance and recommendation of countermeasures. HUMEX-TN-002, 2001.
-
- Guéguinou N, Huin-Schohn C, Bascove M, Bueb JL, Tschirhart E, Legrand-Frossi C, et al. Could spaceflight-associated immune system weakening pre-clude the expansion of human presence beyond Earth's orbit?, J Leukoc Biol. 2009; 86: 1027–1038 doi: 10.1189/jlb.0309167 - DOI - PubMed
-
- Horneck G, Facius R, Reichert M, Rettberg P, Seboldt W, Manzey D, et al. HUMEX, a study on the survivability and adaptation of humans to long-duration exploratory missions, part II: Missions to Mars. In: Advances in Space Research, Mercury, Mars and Saturn, vol. 38; 2006. pp. 752–759 - PubMed
-
- Cogoli A, Bechler B, Müller O, Hunzinger E. Effect of microgravity on lymphocyte activation. In: Biorack on Spacelab D1. European Space Agency, Ed. Paris; 1988, pp. 89–100.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

