P4HA1 mutations cause a unique congenital disorder of connective tissue involving tendon, bone, muscle and the eye
- PMID: 28419360
- PMCID: PMC6075373
- DOI: 10.1093/hmg/ddx110
P4HA1 mutations cause a unique congenital disorder of connective tissue involving tendon, bone, muscle and the eye
Abstract
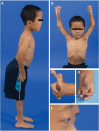
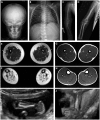
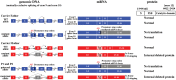
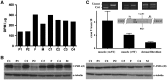
Collagen prolyl 4-hydroxylases (C-P4Hs) play a central role in the formation and stabilization of the triple helical domain of collagens. P4HA1 encodes the catalytic α(I) subunit of the main C-P4H isoenzyme (C-P4H-I). We now report human bi-allelic P4HA1 mutations in a family with a congenital-onset disorder of connective tissue, manifesting as early-onset joint hypermobility, joint contractures, muscle weakness and bone dysplasia as well as high myopia, with evidence of clinical improvement of motor function over time in the surviving patient. Similar to P4ha1 null mice, which die prenatally, the muscle tissue from P1 and P2 was found to have reduced collagen IV immunoreactivity at the muscle basement membrane. Patients were compound heterozygous for frameshift and splice site mutations leading to reduced, but not absent, P4HA1 protein level and C-P4H activity in dermal fibroblasts compared to age-matched control samples. Differential scanning calorimetry revealed reduced thermal stability of collagen in patient-derived dermal fibroblasts versus age-matched control samples. Mutations affecting the family of C-P4Hs, and in particular C-P4H-I, should be considered in patients presenting with congenital connective tissue/myopathy overlap disorders with joint hypermobility, contractures, mild skeletal dysplasia and high myopia.
Published by Oxford University Press 2017. This work is written by US Government employees and is in the public domain in the US.
Figures

References
-
- Kivirikko K.I., Pihlajaniemi T. (1998) Collagen hydroxylases and the protein disulfide isomerase subunit of prolyl 4-hydroxylases. Adv. Enzymol Relat. Areas Mol. Biol., 72, 325–398. - PubMed
-
- Myllyharju J. (2003) Prolyl 4-hydroxylases, the key enzymes of collagen biosynthesis. Matrix Biol., 22, 15–24. - PubMed
-
- Myllyharju J., Kivirikko K.I. (2004) Collagens, modifying enzymes and their mutations in humans, flies and worms. Trends Genet., 20, 33–43. - PubMed
-
- Helaakoski T., Vuori K., Myllyla R., Kivirikko K.I., Pihlajaniemi T. (1989) Molecular cloning of the alpha-subunit of human prolyl 4-hydroxylase: the complete cDNA-derived amino acid sequence and evidence for alternative splicing of RNA transcripts. Proc. Natl. Acad. Sci. USA, 86, 4392–4396. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

