Cardiac troponin T and fast skeletal muscle denervation in ageing
- PMID: 28419739
- PMCID: PMC5659053
- DOI: 10.1002/jcsm.12204
Cardiac troponin T and fast skeletal muscle denervation in ageing
Abstract
Background: Ageing skeletal muscle undergoes chronic denervation, and the neuromuscular junction (NMJ), the key structure that connects motor neuron nerves with muscle cells, shows increased defects with ageing. Previous studies in various species have shown that with ageing, type II fast-twitch skeletal muscle fibres show more atrophy and NMJ deterioration than type I slow-twitch fibres. However, how this process is regulated is largely unknown. A better understanding of the mechanisms regulating skeletal muscle fibre-type specific denervation at the NMJ could be critical to identifying novel treatments for sarcopenia. Cardiac troponin T (cTnT), the heart muscle-specific isoform of TnT, is a key component of the mechanisms of muscle contraction. It is expressed in skeletal muscle during early development, after acute sciatic nerve denervation, in various neuromuscular diseases and possibly in ageing muscle. Yet the subcellular localization and function of cTnT in skeletal muscle is largely unknown.
Methods: Studies were carried out on isolated skeletal muscles from mice, vervet monkeys, and humans. Immunoblotting, immunoprecipitation, and mass spectrometry were used to analyse protein expression, real-time reverse transcription polymerase chain reaction was used to measure gene expression, immunofluorescence staining was performed for subcellular distribution assay of proteins, and electromyographic recording was used to analyse neurotransmission at the NMJ.
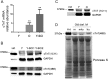
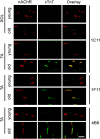
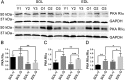
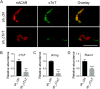
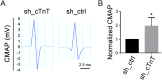
Results: Levels of cTnT expression in skeletal muscle increased with ageing in mice. In addition, cTnT was highly enriched at the NMJ region-but mainly in the fast-twitch, not the slow-twitch, muscle of old mice. We further found that the protein kinase A (PKA) RIα subunit was largely removed from, while PKA RIIα and RIIβ are enriched at, the NMJ-again, preferentially in fast-twitch but not slow-twitch muscle in old mice. Knocking down cTnT in fast skeletal muscle of old mice: (i) increased PKA RIα and reduced PKA RIIα at the NMJ; (ii) decreased the levels of gene expression of muscle denervation markers; and (iii) enhanced neurotransmission efficiency at NMJ.
Conclusions: Cardiac troponin T at the NMJ region contributes to NMJ functional decline with ageing mainly in the fast-twitch skeletal muscle through interfering with PKA signalling. This knowledge could inform useful targets for prevention and therapy of age-related decline in muscle function.
Keywords: Cardiac troponin T; Denervation; Neuromuscular junction; Protein kinase A; Sarcopenia.
© 2017 The Authors. Journal of Cachexia, Sarcopenia and Muscle published by John Wiley & Sons Ltd on behalf of the Society on Sarcopenia, Cachexia and Wasting Disorders.
Figures



Similar articles
-
Alterations in neuromuscular junction morphology with ageing and endurance training modulate neuromuscular transmission and myofibre composition.J Physiol. 2025 Jan;603(1):107-125. doi: 10.1113/JP285143. Epub 2024 Jan 3. J Physiol. 2025. PMID: 38173183 Free PMC article.
-
Effect of denervation on the content of cardiac troponin-T and cardiac troponin-I in rat skeletal muscle.Clin Biochem. 2007 Mar;40(5-6):423-6. doi: 10.1016/j.clinbiochem.2006.12.008. Epub 2007 Jan 13. Clin Biochem. 2007. PMID: 17303103
-
Smoke-induced neuromuscular junction degeneration precedes the fibre type shift and atrophy in chronic obstructive pulmonary disease.J Physiol. 2018 Jul;596(14):2865-2881. doi: 10.1113/JP275558. Epub 2018 May 19. J Physiol. 2018. PMID: 29663403 Free PMC article.
-
Neuromuscular junction transmission failure in aging and sarcopenia: The nexus of the neurological and muscular systems.Ageing Res Rev. 2023 Aug;89:101966. doi: 10.1016/j.arr.2023.101966. Epub 2023 Jun 1. Ageing Res Rev. 2023. PMID: 37270145 Free PMC article. Review.
-
Impact of ageing and disuse on neuromuscular junction and mitochondrial function and morphology: Current evidence and controversies.Ageing Res Rev. 2024 Dec;102:102586. doi: 10.1016/j.arr.2024.102586. Epub 2024 Nov 17. Ageing Res Rev. 2024. PMID: 39557298 Review.
Cited by
-
Regulation of skeletal myogenesis in C2C12 cells through modulation of Pax7, MyoD, and myogenin via different low-frequency electromagnetic field energies.Technol Health Care. 2022;30(S1):371-382. doi: 10.3233/THC-THC228034. Technol Health Care. 2022. PMID: 35124612 Free PMC article.
-
Cardiac alterations following experimental hip fracture - inflammaging as independent risk factor.Front Immunol. 2022 Sep 5;13:895888. doi: 10.3389/fimmu.2022.895888. eCollection 2022. Front Immunol. 2022. PMID: 36131923 Free PMC article.
-
Tissue-Engineered Human Myobundle System as a Platform for Evaluation of Skeletal Muscle Injury Biomarkers.Toxicol Sci. 2020 Jul 1;176(1):124-136. doi: 10.1093/toxsci/kfaa049. Toxicol Sci. 2020. PMID: 32294208 Free PMC article.
-
Focus on cardiac troponin complex: From gene expression to cardiomyopathy.Genes Dis. 2024 Mar 11;11(6):101263. doi: 10.1016/j.gendis.2024.101263. eCollection 2024 Nov. Genes Dis. 2024. PMID: 39211905 Free PMC article. Review.
-
The emerging role of the sympathetic nervous system in skeletal muscle motor innervation and sarcopenia.Ageing Res Rev. 2021 May;67:101305. doi: 10.1016/j.arr.2021.101305. Epub 2021 Feb 18. Ageing Res Rev. 2021. PMID: 33610815 Free PMC article. Review.
References
-
- el‐Saleh SC, Warber KD, Potter JD. The role of tropomyosin‐troponin in the regulation of skeletal muscle contraction. J Muscle Res Cell Motil 1986;7:387–404. - PubMed
-
- Asumda FZ, Chase PB. Nuclear cardiac troponin and tropomyosin are expressed early in cardiac differentiation of rat mesenchymal stem cells. Differentiation 2012;83:106–115. - PubMed
-
- Sahota VK, Grau BF, Mansilla A, Ferrus A. Troponin I and tropomyosin regulate chromosomal stability and cell polarity. J Cell Sci 2009;122:2623–2631. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

