Nonalcoholic Fatty Liver Disease and Insulin Resistance: New Insights and Potential New Treatments
- PMID: 28420094
- PMCID: PMC5409726
- DOI: 10.3390/nu9040387
Nonalcoholic Fatty Liver Disease and Insulin Resistance: New Insights and Potential New Treatments
Abstract
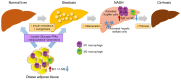
Nonalcoholic fatty liver disease (NAFLD) is one of the most common chronic liver disorders worldwide. It is associated with clinical states such as obesity, insulin resistance, and type 2 diabetes, and covers a wide range of liver changes, ranging from simple steatosis to non-alcoholic steatohepatitis (NASH), liver cirrhosis, and hepatocellular carcinoma. Metabolic disorders, such as lipid accumulation, insulin resistance, and inflammation, have been implicated in the pathogenesis of NAFLD, but the underlying mechanisms, including those that drive disease progression, are not fully understood. Both innate and recruited immune cells mediate the development of insulin resistance and NASH. Therefore, modifying the polarization of resident and recruited macrophage/Kupffer cells is expected to lead to new therapeutic strategies in NAFLD. Oxidative stress is also pivotal for the progression of NASH, which has generated interest in carotenoids as potent micronutrient antioxidants in the treatment of NAFLD. In addition to their antioxidative function, carotenoids regulate macrophage/Kupffer cell polarization and thereby prevent NASH progression. In this review, we summarize the molecular mechanisms involved in the pathogenesis of NAFLD, including macrophage/Kupffer cell polarization, and disturbed hepatic function in NAFLD. We also discuss dietary antioxidants, such as β-cryptoxanthin and astaxanthin, that may be effective in the prevention or treatment of NAFLD.
Keywords: NAFLD/NASH; antioxidants; astaxanthin; chemokine; fibrosis; inflammation; insulin resistance; macrophage/Kupffer cells; β-cryptoxanthin.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- Yatsuji S., Hashimoto E., Tobari M., Taniai M., Tokushige K., Shiratori K. Clinical features and outcomes of cirrhosis due to non-alcoholic steatohepatitis compared with cirrhosis caused by chronic hepatitis C. J. Gastroenterol. Hepatol. 2009;24:248–254. doi: 10.1111/j.1440-1746.2008.05640.x. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

