Electrochemical Genosensing of Circulating Biomarkers
- PMID: 28420103
- PMCID: PMC5424743
- DOI: 10.3390/s17040866
Electrochemical Genosensing of Circulating Biomarkers
Abstract
Management and prognosis of diseases requires the measurement in non- or minimally invasively collected samples of specific circulating biomarkers, consisting of any measurable or observable factors in patients that indicate normal or disease-related biological processes or responses to therapy. Therefore, on-site, fast and accurate determination of these low abundance circulating biomarkers in scarcely treated body fluids is of great interest for health monitoring and biological applications. In this field, electrochemical DNA sensors (or genosensors) have demonstrated to be interesting alternatives to more complex conventional strategies. Currently, electrochemical genosensors are considered very promising analytical tools for this purpose due to their fast response, low cost, high sensitivity, compatibility with microfabrication technology and simple operation mode which makes them compatible with point-of-care (POC) testing. In this review, the relevance and current challenges of the determination of circulating biomarkers related to relevant diseases (cancer, bacterial and viral infections and neurodegenerative diseases) are briefly discussed. An overview of the electrochemical nucleic acid-based strategies developed in the last five years for this purpose is given to show to both familiar and non-expert readers the great potential of these methodologies for circulating biomarker determination. After highlighting the main features of the reported electrochemical genosensing strategies through the critical discussion of selected examples, a conclusions section points out the still existing challenges and future directions in this field.
Keywords: bacterial and viral infections; cancer; circulating biomarkers; electrochemical genosensors; neurodegenerative diseases.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

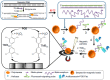
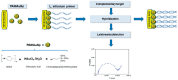
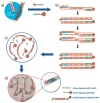
Similar articles
-
Multiplexed Electrochemical Immunosensors for Clinical Biomarkers.Sensors (Basel). 2017 Apr 27;17(5):965. doi: 10.3390/s17050965. Sensors (Basel). 2017. PMID: 28448466 Free PMC article. Review.
-
Non-Invasive Breast Cancer Diagnosis through Electrochemical Biosensing at Different Molecular Levels.Sensors (Basel). 2017 Aug 31;17(9):1993. doi: 10.3390/s17091993. Sensors (Basel). 2017. PMID: 28858236 Free PMC article. Review.
-
Pushing the limits of electrochemistry toward challenging applications in clinical diagnosis, prognosis, and therapeutic action.Chem Commun (Camb). 2019 Feb 26;55(18):2563-2592. doi: 10.1039/c8cc08815b. Chem Commun (Camb). 2019. PMID: 30688320 Review.
-
Electrochemical Nucleic Acid-Based Biosensing of Drugs of Abuse and Pharmaceuticals.Curr Med Chem. 2018;25(33):4102-4118. doi: 10.2174/0929867324666171121103156. Curr Med Chem. 2018. PMID: 29165065 Review.
-
Revisiting Electrochemical Biosensing in the 21st Century Society for Inflammatory Cytokines Involved in Autoimmune, Neurodegenerative, Cardiac, Viral and Cancer Diseases.Sensors (Basel). 2020 Dec 30;21(1):189. doi: 10.3390/s21010189. Sensors (Basel). 2020. PMID: 33396710 Free PMC article. Review.
Cited by
-
Noninvasive evaluation of hemodynamics and light scattering property during two-stage mouse cutaneous carcinogenesis based on multispectral diffuse reflectance images at isosbestic wavelengths of hemoglobin.J Biomed Opt. 2019 Jan;24(3):1-11. doi: 10.1117/1.JBO.24.3.031020. J Biomed Opt. 2019. PMID: 30635994 Free PMC article.
-
TBISTAT: An open-source, wireless portable, electrochemical impedance spectroscopy capable potentiostat for the point-of-care detection of S100B in plasma samples.PLoS One. 2022 Feb 7;17(2):e0263738. doi: 10.1371/journal.pone.0263738. eCollection 2022. PLoS One. 2022. PMID: 35130295 Free PMC article.
-
Toward waterborne protozoa detection using sensing technologies.Front Microbiol. 2023 Feb 24;14:1118164. doi: 10.3389/fmicb.2023.1118164. eCollection 2023. Front Microbiol. 2023. PMID: 36910193 Free PMC article. Review.
-
Nano-engineered screen-printed electrodes: A dynamic tool for detection of viruses.Trends Analyt Chem. 2021 Oct;143:116374. doi: 10.1016/j.trac.2021.116374. Epub 2021 Jun 21. Trends Analyt Chem. 2021. PMID: 34177011 Free PMC article. Review.
-
The Role of Electrochemical Sensors in Enhancing HIV Detection.Curr HIV Res. 2025;23(1):2-13. doi: 10.2174/011570162X363311250206045837. Curr HIV Res. 2025. PMID: 39950463 Review.
References
-
- Costa Rama E., Costa-García A. Screen-Printed Electrochemical Immunosensors for the Detection of Cancer and Cardiovascular Biomarkers. Electroanalysis. 2016;28:1700–1715. doi: 10.1002/elan.201600126. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

