Clinical Outcomes of 217 Patients with Acute Erythroleukemia According to Treatment Type and Line: A Retrospective Multinational Study
- PMID: 28420120
- PMCID: PMC5412421
- DOI: 10.3390/ijms18040837
Clinical Outcomes of 217 Patients with Acute Erythroleukemia According to Treatment Type and Line: A Retrospective Multinational Study
Abstract
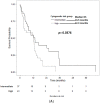
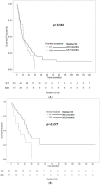
Acute erythroleukemia (AEL) is a rare disease typically associated with a poor prognosis. The median survival ranges between 3-9 months from initial diagnosis. Hypomethylating agents (HMAs) have been shown to prolong survival in patients with myelodysplastic syndromes (MDS) and AML, but there is limited data of their efficacy in AEL. We collected data from 210 AEL patients treated at 28 international sites. Overall survival (OS) and PFS were estimated using the Kaplan-Meier method and the log-rank test was used for subgroup comparisons. Survival between treatment groups was compared using the Cox proportional hazards regression model. Eighty-eight patients were treated with HMAs, 44 front line, and 122 with intensive chemotherapy (ICT). ICT led to a higher overall response rate (complete or partial) compared to first-line HMA (72% vs. 46.2%, respectively; p ≤ 0.001), but similar progression-free survival (8.0 vs. 9.4 months; p = 0.342). Overall survival was similar for ICT vs. HMAs (10.5 vs. 13.7 months; p = 0.564), but patients with high-risk cytogenetics treated with HMA first-line lived longer (7.5 for ICT vs. 13.3 months; p = 0.039). Our results support the therapeutic value of HMA in AEL.
Keywords: acute erythroleukemia; azacitidine; decitabine.
Conflict of interest statement
Antonio M. Almeida: speaker and advisory board for Celgene; Arjan A. Van De Loosdrecht: speaker and advisory board Celgene, advisory board Novartis; Jamile Shammo: Received research funding and honoraria for speaking engagements and consultancy from Celgene; Peter Valent received a research grant and speaker´s honoraria from Celgene and served as an advisory board member for Celgene. Fernando Ramos: Honoraria/Consultation fees for Celgene, Janssen, Amgen, Novatis, Pfizer, Glaxo-Smith-Kline, Merck-Sharp & Dohme. Maria Diez Campelo: speaker, research founding and advisory boards for Celgene.
Figures


Similar articles
-
Myelodysplastic syndromes following therapy with hypomethylating agents (HMAs): development of acute erythroleukemia may not influence assessment of treatment response.Leuk Lymphoma. 2016;57(4):812-9. doi: 10.3109/10428194.2015.1079318. Epub 2016 Mar 4. Leuk Lymphoma. 2016. PMID: 26293512
-
An analysis of 97 previously diagnosed de novo adult acute erythroid leukemia patients following the 2016 revision to World Health Organization classification.BMC Cancer. 2017 Aug 9;17(1):534. doi: 10.1186/s12885-017-3528-6. BMC Cancer. 2017. PMID: 28793875 Free PMC article.
-
Predictive factors for response and survival in elderly acute myeloid leukemia patients treated with hypomethylating agents: a real-life experience.Ann Hematol. 2020 Oct;99(10):2405-2416. doi: 10.1007/s00277-020-04217-w. Epub 2020 Aug 19. Ann Hematol. 2020. PMID: 32813071
-
Clinical activity, pharmacokinetics, and pharmacodynamics of oral hypomethylating agents for myelodysplastic syndromes/neoplasms and acute myeloid leukemia: A multidisciplinary review.J Oncol Pharm Pract. 2024 Jun;30(4):721-736. doi: 10.1177/10781552241238979. Epub 2024 Mar 21. J Oncol Pharm Pract. 2024. PMID: 38509812 Free PMC article. Review.
-
Guadecitabine (SGI-110): an investigational drug for the treatment of myelodysplastic syndrome and acute myeloid leukemia.Expert Opin Investig Drugs. 2019 Oct;28(10):835-849. doi: 10.1080/13543784.2019.1667331. Epub 2019 Sep 19. Expert Opin Investig Drugs. 2019. PMID: 31510809 Review.
Cited by
-
Genomic subtyping and therapeutic targeting of acute erythroleukemia.Nat Genet. 2019 Apr;51(4):694-704. doi: 10.1038/s41588-019-0375-1. Epub 2019 Mar 29. Nat Genet. 2019. PMID: 30926971 Free PMC article.
-
Survival after Pure (Acute) Erythroid Leukemia in the United States: A SEER-Based Study.Cancers (Basel). 2023 Aug 3;15(15):3941. doi: 10.3390/cancers15153941. Cancers (Basel). 2023. PMID: 37568757 Free PMC article.
-
Acute Myeloid Leukaemia: New Targets and Therapies.Int J Mol Sci. 2017 Nov 30;18(12):2577. doi: 10.3390/ijms18122577. Int J Mol Sci. 2017. PMID: 29189736 Free PMC article.
-
Molecular Landscapes and Models of Acute Erythroleukemia.Hemasphere. 2021 Apr 21;5(5):e558. doi: 10.1097/HS9.0000000000000558. eCollection 2021 May. Hemasphere. 2021. PMID: 33898929 Free PMC article. Review.
-
Randomized phase-II trial evaluating induction therapy with idarubicin and etoposide plus sequential or concurrent azacitidine and maintenance therapy with azacitidine.Leukemia. 2019 Aug;33(8):1923-1933. doi: 10.1038/s41375-019-0395-y. Epub 2019 Feb 6. Leukemia. 2019. PMID: 30728457 Free PMC article. Clinical Trial.
References
-
- Zuo Z., Polski J.M., Kasyan A., Medeiros L.J. Acute erythroid leukemia. Arch. Pathol. Lab. Med. 2010;134:1261–1270. - PubMed
-
- Kasyan A., Medeiros L.J., Zuo Z., Santos F.P., Ravandi-Kashani F., Miranda R., Vadhan-Raj S., Koeppen H., Bueso-Ramos C.E. Acute erythroid leukemia as defined in the World Health Organization classification is a rare and pathogenetically heterogeneous disease. Mod. Pathol. 2010;23:1113–1126. doi: 10.1038/modpathol.2010.96. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

