Beneficial Effects of Melatonin on the In Vitro Maturation of Sheep Oocytes and Its Relation to Melatonin Receptors
- PMID: 28420163
- PMCID: PMC5412418
- DOI: 10.3390/ijms18040834
Beneficial Effects of Melatonin on the In Vitro Maturation of Sheep Oocytes and Its Relation to Melatonin Receptors
Abstract
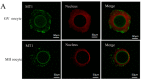
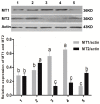
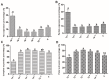
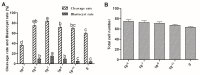
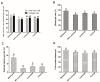
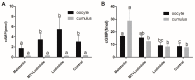
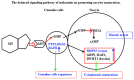
(1) Background: The binding sites of melatonin, as a multifunctional molecule, have been identified in human, porcine, and bovine samples. However, the binding sites and mechanisms of melatonin have not been reported in sheep; (2) Methods: Cumulus-oocyte complexes (COCs) were cultured in TCM-199 supplemented with melatonin at concentrations of 0, 10-3, 10-5, 10-7, 10-9, and 10-11 M. Melatonin receptors (MT1 and MT2) were evaluated via immunofluorescence and Western blot. The effects of melatonin on cumulus cell expansion, nuclear maturation, embryo development, and related gene (GDF9, DNMT1, PTX3, HAS2, and EGFR) expression were investigated. The level of cyclic adenosine monophosphate (cAMP) and cyclic guanosine monophosphate (cGMP) were evaluated in oocytes and cumulus, respectively; (3) Results: Both MT1 and MT2 were expressed in oocytes, cumulus cells, and granulosa cells. Melatonin with a concentration of 10-7 M significantly enhanced the rates of nuclear maturation, cumulus cells expansion, cleavage, and blastocyst. Melatonin enhanced the expression of BMP15 in oocytes and of PTX3, HAS2, and EGFR in cumulus cells. Melatonin decreased the cAMP level of oocytes but enhanced the cGMP level in oocytes and cumulus cells; (4) Conclusion: The higher presence of MT1 in GV cumulus cells and the beneficial effects of melatonin indicated that its roles in regulating sheep oocyte maturation may be mediated mainly by the MT1 receptor.
Keywords: MT1/MT2; meioctic maturation; melatonin; oocyte; sheep.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

