The Angiogenesis Inhibitor ALS-L1023 from Lemon-Balm Leaves Attenuates High-Fat Diet-Induced Nonalcoholic Fatty Liver Disease through Regulating the Visceral Adipose-Tissue Function
- PMID: 28420164
- PMCID: PMC5412430
- DOI: 10.3390/ijms18040846
The Angiogenesis Inhibitor ALS-L1023 from Lemon-Balm Leaves Attenuates High-Fat Diet-Induced Nonalcoholic Fatty Liver Disease through Regulating the Visceral Adipose-Tissue Function
Abstract
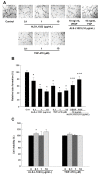
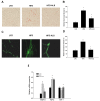
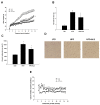
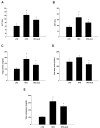
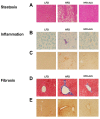
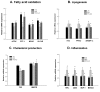
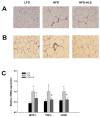
Similar to neoplastic tissues, growth and development of adipose tissue are thought to be angiogenesis-dependent. Since visceral adipose tissue (VAT) is associated with development and progression of nonalcoholic fatty liver disease (NAFLD), we hypothesized that angiogenesis inhibition would attenuate obesity-induced NAFLD. We fed C57BL/6J mice a low-fat diet (LFD, chow 10% kcal fat), a high-fat diet (HFD, 45% kcal fat) or HFD supplemented with the lemon-balm extract ALS-L1023 (HFD-ALS) for 15 weeks. ALS-L1023 reduced endothelial cell-tube formation in vitro. HFD increased VAT angiogenesis and induced weight gains including body weight, VAT mass and visceral adipocyte size compared with LFD. However, HFD-ALS led to weight reductions without affecting calorie intake compared with HFD. HFD-ALS also reduced serum ALT and AST levels and improved lipid metabolism. HFD-ALS suppressed steatosis, infiltration of inflammatory cells, and accumulation of collagen in livers. HFD-ALS modulated hepatic expression of genes involved in lipid metabolism, inflammation, fibrosis, antioxidation, and apoptosis. Concomitantly, analysis of VAT function revealed that HFD-ALS led to fewer CD68-positive macrophage numbers and lower expression of inflammatory cytokines compared with HFD. Our findings show that the anti-angiogenic herbal extract ALS-L1023 attenuates NAFLD by targeting VAT during obesity, suggesting that angiogenesis inhibitors could aid in the treatment and prevention of obesity-induced human NAFLD.
Keywords: Melissa officinalis; herbal medicine; visceral adipose inflammation; visceral obesity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Folkman J. Tumor angiogenesis. Adv. Cancer Res. 1985;43:175–203. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

