Hypaphorine Attenuates Lipopolysaccharide-Induced Endothelial Inflammation via Regulation of TLR4 and PPAR-γ Dependent on PI3K/Akt/mTOR Signal Pathway
- PMID: 28420166
- PMCID: PMC5412428
- DOI: 10.3390/ijms18040844
Hypaphorine Attenuates Lipopolysaccharide-Induced Endothelial Inflammation via Regulation of TLR4 and PPAR-γ Dependent on PI3K/Akt/mTOR Signal Pathway
Abstract
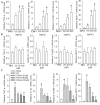
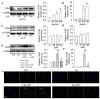
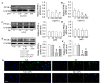
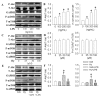
Endothelial lesion response to injurious stimuli is a necessary step for initiating inflammatory cascades in blood vessels. Hypaphorine (Hy) from different marine sources is shown to exhibit anti-inflammatory properties. However, the potential roles and possible molecular mechanisms of Hy in endothelial inflammation have yet to be fully clarified. We showed that Hy significantly inhibited the positive effects of lipopolysaccharide (LPS) on pro-inflammatory cytokines expressions, including tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), monocyte chemoattractant protein 1 (MCP-1) and vascular cellular adhesion molecule-1 (VCAM-1), as well as induction of the phosphorylation of Akt and mTOR in HMEC-1 cells. The downregulated peroxisome proliferator-activated receptor γ (PPAR-γ) and upregulated toll-like receptor 4 (TLR4) expressions in LPS-challenged endothelial cells were prevented by Hy. Inhibition of both PI3K and mTOR reversed LPS-stimulated increases in TLR4 expressions and decreases in PPAR-γ levels. Genetic silencing of TLR4 or PPAR-γ agonist pioglitazone obviously abrogated the levels of pro-inflammatory cytokines in LPS-treated HMEC-1 cells. These results suggest that Hy may exert anti-inflammatory actions through the regulation of TLR4 and PPAR-γ dependent on PI3K/Akt/mTOR signal pathways. Hy may be considered as a therapeutic agent that can potentially relieve or ameliorate endothelial inflammation-associated diseases.
Keywords: LPS; PPAR-γ; TLR4; endothelial cells; inflammation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Interactions of TLR4 and PPARγ, Dependent on AMPK Signalling Pathway Contribute to Anti-Inflammatory Effects of Vaccariae Hypaphorine in Endothelial Cells.Cell Physiol Biochem. 2017;42(3):1227-1239. doi: 10.1159/000478920. Epub 2017 Jul 3. Cell Physiol Biochem. 2017. PMID: 28683454
-
Ginkgolide A inhibits lipopolysaccharide-induced inflammatory response in human coronary artery endothelial cells via downregulation of TLR4-NF-κB signaling through PI3K/Akt pathway.Pharmazie. 2016 Oct 1;71(10):588-591. doi: 10.1691/ph.2016.6576. Pharmazie. 2016. PMID: 29441927
-
Melatonin modulates TLR4-mediated inflammatory genes through MyD88- and TRIF-dependent signaling pathways in lipopolysaccharide-stimulated RAW264.7 cells.J Pineal Res. 2012 Nov;53(4):325-34. doi: 10.1111/j.1600-079X.2012.01002.x. Epub 2012 Apr 27. J Pineal Res. 2012. PMID: 22537289
-
Targeted abrogation of diverse signal transduction cascades by emodin for the treatment of inflammatory disorders and cancer.Cancer Lett. 2013 Dec 1;341(2):139-49. doi: 10.1016/j.canlet.2013.08.023. Epub 2013 Aug 17. Cancer Lett. 2013. PMID: 23962559 Review.
-
Link between microbiota and hypertension: Focus on LPS/TLR4 pathway in endothelial dysfunction and vascular inflammation, and therapeutic implication of probiotics.Biomed Pharmacother. 2021 May;137:111334. doi: 10.1016/j.biopha.2021.111334. Epub 2021 Feb 5. Biomed Pharmacother. 2021. PMID: 33556874 Review.
Cited by
-
Bilobalide attenuates lipopolysaccharide‑induced HepG2 cell injury by inhibiting TLR4‑NF‑κB signaling via the PI3K/Akt pathway.Exp Ther Med. 2023 Nov 22;27(1):24. doi: 10.3892/etm.2023.12312. eCollection 2024 Jan. Exp Ther Med. 2023. PMID: 38125341 Free PMC article.
-
Activation of STAT3 is a key event in TLR4 signaling-mediated melanoma progression.Cell Death Dis. 2020 Apr 20;11(4):246. doi: 10.1038/s41419-020-2440-1. Cell Death Dis. 2020. PMID: 32312954 Free PMC article.
-
Role of Different Blood Purification Nursing Models in Uremic Patients: A Preliminary Report.Med Sci Monit. 2018 Sep 28;24:6873-6881. doi: 10.12659/MSM.910877. Med Sci Monit. 2018. PMID: 30264774 Free PMC article.
-
The synergistic ameliorative activity of peroxisome proliferator-activated receptor-alpha and gamma agonists, fenofibrate and pioglitazone, on hippocampal neurodegeneration in a rat model of insulin resistance.Ibrain. 2022 Aug 8;8(3):251-263. doi: 10.1002/ibra.12059. eCollection 2022 Fall. Ibrain. 2022. PMID: 37786742 Free PMC article.
-
Hepatoprotective effects of Niudali (Callerya speciosa) root aqueous extracts against tetrachloromethane-induced acute liver injury and inflammation.Food Sci Nutr. 2023 Aug 17;11(11):7026-7038. doi: 10.1002/fsn3.3626. eCollection 2023 Nov. Food Sci Nutr. 2023. PMID: 37970412 Free PMC article.
References
-
- Cines D.B., Pollak E.S., Buck C.A., Loscalzo J., Zimmerman G.A., McEver R.P., Pober J.S., Wick T.M., Konkle B.A., Schwartz B.S., et al. Endothelial cells in physiology and in the pathophysiology of vascular disorders. Blood. 1998;91:3527–3561. - PubMed
-
- Gray S.P., Jandeleit-Dahm K.A. The role of NADPH oxidase in vascular disease—Hypertension, atherosclerosis & stroke. Curr. Pharm. Des. 2015;21:5933–5944. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

