Treatment and Prevention of Bleeds in Haemophilia Patients with Inhibitors to Factor VIII/IX
- PMID: 28420167
- PMCID: PMC5406778
- DOI: 10.3390/jcm6040046
Treatment and Prevention of Bleeds in Haemophilia Patients with Inhibitors to Factor VIII/IX
Abstract
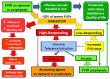
The development of alloantibodies neutralising therapeutically administered factor (F) VIII/IX (inhibitors) is currently the most severe complication of the treatment of haemophilia. When persistent and at a high titre, inhibitors preclude the standard replacement treatment with FVIII/FIX concentrates, making patients' management challenging. Indeed, the efficacy of bypassing agents, i.e., activated prothrombin complex concentrates (aPCC) and recombinant activated factor VII (rFVIIa), needed to overcome the haemostatic interference of the inhibitor, is not comparable to that of factor concentrates. In addition, the therapeutical response is unpredictable, with a relevant inter-individual and even intra-individual variability, and no laboratory assay is validated to monitor the efficacy and safety of the treatment. As a result, inhibitor patients have a worse joint status and quality of life compared to inhibitor-free subjects and the eradication of the inhibitor by immune tolerance induction is the preeminent therapeutic goal, particularly in children. However, over the last decades, treatment with bypassing agents has been optimised, allowing home treatment and the individualisation of regimens aimed at improving clinical outcomes. In this respect, a growing body of evidence supports the efficacy of prophylaxis with both bypassing agents in reducing bleeding rates and improving the quality of life, although the impact on long-term outcomes (in particular on preventing/reducing joint deterioration) is still unknown. This review offers an update on the current knowledge and practice of the use of bypassing agents in haemophiliacs with inhibitors, as well as on debated issues and unmet needs in this challenging setting.
Keywords: bleeding; bypassing therapy; haemophilia; inhibitors; prophylaxis.
Conflict of interest statement
A.R. has been a member of advisory boards for Baxter, Bayer, Novo Nordisk, Pfizer and Sobi, acted as a paid consultant for Bayer, CSL Behring, Kedrion, Novo Nordisk and Pfizer and received fees as a speaker in meetings organised by Baxter, Bayer, CSL Behring, Novo Nordisk and Pfizer. M.F. has acted as a paid consultant for Bayer, CSL Behring, Novo Nordisk and Kedrion. A.C. has received fees as a consultant or advisory board member from Bayer, CSL Behring, Novo Nordisk, Octapharma and Sobi.
Figures
References
-
- Gouw S.C., van den Berg H.M., Fischer K., Auerswald G., Carcao M., Chalmers E., Chambost H., Kurnik K., Liesner R., Petrini P., et al. Intensity of factor VIII treatment and inhibitor development in children with severe hemophilia A: The RODIN study. Blood. 2013;121:4046–4055. doi: 10.1182/blood-2012-09-457036. - DOI - PubMed
-
- Hay C.R., Palmer B., Chalmers E., Liesner R., Maclean R., Rangarajan S., Williams M., Collins P.W., United Kingdom Haemophilia Centre Doctors’ Organisation (UKHCDO) Incidence of factor VIII inhibitors throughout life in severe hemophilia A in the United Kingdom. Blood. 2011;117:6367–6370. doi: 10.1182/blood-2010-09-308668. - DOI - PubMed
-
- Gouw S.C., Fijnvandraat K. Identifying non genetic risk factors for inhibitor development in severe hemophilia A. Semin. Thromb. Hemost. 2013;39:740–751. - PubMed
-
- Astermark J., Altisent C., Batorova A., Diniz M.J., Gringeri A., Holme P.A., Karafoulidou A., Lopez-Fernández M.F., Reipert B.M., Rocino A., et al. Non-genetic risk factors and the development of inhibitors in haemophilia: A comprehensive review and consensus report. Haemophilia. 2010;16:747–766. doi: 10.1111/j.1365-2516.2010.02231.x. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

