Fructose Intake, Serum Uric Acid, and Cardiometabolic Disorders: A Critical Review
- PMID: 28420204
- PMCID: PMC5409734
- DOI: 10.3390/nu9040395
Fructose Intake, Serum Uric Acid, and Cardiometabolic Disorders: A Critical Review
Abstract
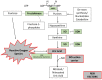
There is a direct relationship between fructose intake and serum levels of uric acid (UA), which is the final product of purine metabolism. Recent preclinical and clinical evidence suggests that chronic hyperuricemia is an independent risk factor for hypertension, metabolic syndrome, and cardiovascular disease. It is probably also an independent risk factor for chronic kidney disease, Type 2 diabetes, and cognitive decline. These relationships have been observed for high serum UA levels (>5.5 mg/dL in women and >6 mg/dL in men), but also for normal to high serum UA levels (5-6 mg/dL). In this regard, blood UA levels are much higher in industrialized countries than in the rest of the world. Xanthine-oxidase inhibitors can reduce UA and seem to minimize its negative effects on vascular health. Other dietary and pathophysiological factors are also related to UA production. However, the role of fructose-derived UA in the pathogenesis of cardiometabolic disorders has not yet been fully clarified. Here, we critically review recent research on the biochemistry of UA production, the relationship between fructose intake and UA production, and how this relationship is linked to cardiometabolic disorders.
Keywords: cardiometabolic disorders; epidemiology; fructose; pathophysiology; uric acid; xanthine oxidase.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Nakagawa T., Hu H., Zharikov S., Tuttle K.R., Short R.A., Glushakova O., Ouyang X., Feig D.I., Block E.R., Herrera-Acosta J., et al. A causal role for uric acid in fructose-induced metabolic syndrome. Am. J. Physiol. Renal. Physiol. 2006;290:F625–F631. doi: 10.1152/ajprenal.00140.2005. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

