Optimizing veteran-centered prostate cancer survivorship care: study protocol for a randomized controlled trial
- PMID: 28420419
- PMCID: PMC5395886
- DOI: 10.1186/s13063-017-1925-4
Optimizing veteran-centered prostate cancer survivorship care: study protocol for a randomized controlled trial
Abstract
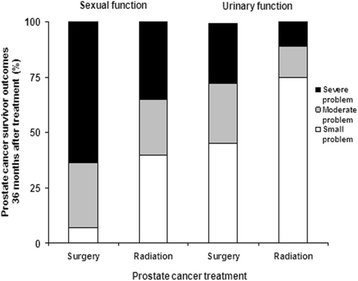
Background: Although prostate cancer is the most common cancer among veterans receiving care in the Veterans Health Administration (VA), more needs to be done to understand and improve survivorship care for this large population. This study, funded by VA Health Services Research & Development (HSR&D), seeks to address the need to improve patient-centered survivorship care for veterans with prostate cancer.
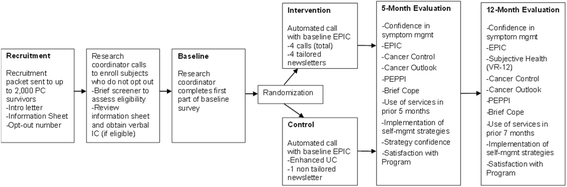
Methods/design: This is a two-armed randomized controlled trial (RCT) with a target enrollment of up to 325 prostate cancer survivors per study arm (total anticipated n = 600). Patients will be recruited from four VA sites. Patient eligibility criteria include age range of 40-80 years, one to ten years post-treatment, and currently experiencing prostate cancer symptom burden. We will compare the "Building Your New Normal" program, a personally-tailored automated telephone symptom management intervention for improving symptom self-management to usual care enhanced with a non-tailored newsletter about symptom management. Primary outcomes include changes in symptom burden, bother, and health services utilization at five and 12 months after enrollment. Secondary outcomes include long-term psychosocial outcomes (e.g. subjective health, perceived cancer control). We will use multivariable regression analysis to evaluate the impact of the intervention on primary and secondary outcomes. We will conduct a process evaluation to understand the effective intervention components and explore possibilities for broader implementation and dissemination.
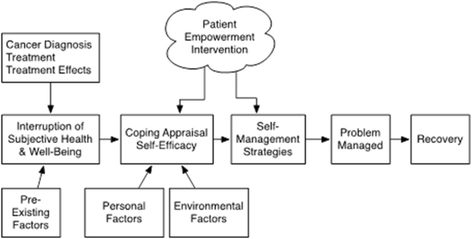
Discussion: Our central hypothesis is that intervention group participants will have improved and more confident symptom self-management and prostate cancer quality of life following the intervention and that these outcomes will translate to more efficient use of health services. The study results will provide much needed information about how to optimize the quality of care, and life, of veteran prostate cancer survivors.
Trial registration: ClinicalTrials.gov ID NCT01900561 ; Registered on 22 July 2013.
Keywords: Health services; Prostatic neoplasms; Quality of life; Self-care; Self-management; Survivorship; Veterans.
Figures
References
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical