Dexamethasone versus standard treatment for postoperative nausea and vomiting in gastrointestinal surgery: randomised controlled trial (DREAMS Trial)
- PMID: 28420629
- PMCID: PMC5482348
- DOI: 10.1136/bmj.j1455
Dexamethasone versus standard treatment for postoperative nausea and vomiting in gastrointestinal surgery: randomised controlled trial (DREAMS Trial)
Abstract
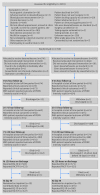
Objectives To determine whether preoperative dexamethasone reduces postoperative vomiting in patients undergoing elective bowel surgery and whether it is associated with other measurable benefits during recovery from surgery, including quicker return to oral diet and reduced length of stay.Design Pragmatic two arm parallel group randomised trial with blinded postoperative care and outcome assessment.Setting 45 UK hospitals.Participants 1350 patients aged 18 or over undergoing elective open or laparoscopic bowel surgery for malignant or benign pathology.Interventions Addition of a single dose of 8 mg intravenous dexamethasone at induction of anaesthesia compared with standard care.Main outcome measures Primary outcome: reported vomiting within 24 hours reported by patient or clinician.
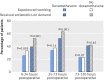
Secondary outcomes: vomiting with 72 and 120 hours reported by patient or clinician; use of antiemetics and postoperative nausea and vomiting at 24, 72, and 120 hours rated by patient; fatigue and quality of life at 120 hours or discharge and at 30 days; time to return to fluid and food intake; length of hospital stay; adverse events.Results 1350 participants were recruited and randomly allocated to additional dexamethasone (n=674) or standard care (n=676) at induction of anaesthesia. Vomiting within 24 hours of surgery occurred in 172 (25.5%) participants in the dexamethasone arm and 223 (33.0%) allocated standard care (number needed to treat (NNT) 13, 95% confidence interval 5 to 22; P=0.003). Additional postoperative antiemetics were given (on demand) to 265 (39.3%) participants allocated dexamethasone and 351 (51.9%) allocated standard care (NNT 8, 5 to 11; P<0.001). Reduction in on demand antiemetics remained up to 72 hours. There was no increase in complications.Conclusions Addition of a single dose of 8 mg intravenous dexamethasone at induction of anaesthesia significantly reduces both the incidence of postoperative nausea and vomiting at 24 hours and the need for rescue antiemetics for up to 72 hours in patients undergoing large and small bowel surgery, with no increase in adverse events.Trial registration EudraCT (2010-022894-32) and ISRCTN (ISRCTN21973627).
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://group.bmj.com/group/rights-licensing/permissions.
Conflict of interest statement
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare: no support from any organisation for the submitted work beyond the study grants listed above; no financial relationships with any organisations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work.
Figures

References
-
- Gan TJ. Postoperative nausea and vomiting--can it be eliminated?JAMA 2002;287:1233-6. 10.1001/jama.287.10.1233 pmid:11886298. - DOI - PubMed
-
- Cohen MM, Duncan PG, DeBoer DP, Tweed WA. The postoperative interview: assessing risk factors for nausea and vomiting. Anesth Analg 1994;78:7-16. 10.1213/00000539-199401000-00004 pmid:8267183. - DOI - PubMed
-
- Macario A, Weinger M, Carney S, Kim A. Which clinical anesthesia outcomes are important to avoid? The perspective of patients. Anesth Analg 1999;89:652-8.pmid:10475299. - PubMed
-
- Apfel CC, Heidrich FM, Jukar-Rao S, et al. Evidence-based analysis of risk factors for postoperative nausea and vomiting. Br J Anaesth 2012;109:742-53. 10.1093/bja/aes276 pmid:23035051. - DOI - PubMed
-
- Ehrlich A, Wagner B, Kairaluoma M, Mecklin JP, Kautiainen H, Kellokumpu I. Evaluation of a fast-track protocol for patients undergoing colorectal surgery. Scand J Surg 2014;103:182-8. 10.1177/1457496913516295 pmid:24694778. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
