Cancer cell metabolism: the essential role of the nonessential amino acid, glutamine
- PMID: 28420743
- PMCID: PMC5430235
- DOI: 10.15252/embj.201696151
Cancer cell metabolism: the essential role of the nonessential amino acid, glutamine
Abstract
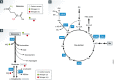
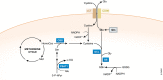
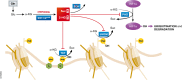
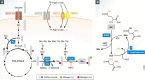
Biochemistry textbooks and cell culture experiments seem to be telling us two different things about the significance of external glutamine supply for mammalian cell growth and proliferation. Despite the fact that glutamine is a nonessential amino acid that can be synthesized by cells from glucose-derived carbons and amino acid-derived ammonia, most mammalian cells in tissue culture cannot proliferate or even survive in an environment that does not contain millimolar levels of glutamine. Not only are the levels of glutamine in standard tissue culture media at least ten-fold higher than other amino acids, but glutamine is also the most abundant amino acid in the human bloodstream, where it is assiduously maintained at approximately 0.5 mM through a combination of dietary uptake, de novo synthesis, and muscle protein catabolism. The complex metabolic logic of the proliferating cancer cells' appetite for glutamine-which goes far beyond satisfying their protein synthesis requirements-has only recently come into focus. In this review, we examine the diversity of biosynthetic and regulatory uses of glutamine and their role in proliferation, stress resistance, and cellular identity, as well as discuss the mechanisms that cells utilize in order to adapt to glutamine limitation.
Keywords: cancer; glutamine metabolism; proliferation; stress response.
© 2017 The Authors.
Figures
References
-
- Ahluwalia GS, Grem JL, Hao Z, Cooney DA (1990) Metabolism and action of amino acid analog anti‐cancer agents. Pharmacol Ther 46: 243–271 - PubMed
-
- Balss J, Meyer J, Mueller W, Korshunov A, Hartmann C, von Deimling A (2008) Analysis of the IDH1 codon 132 mutation in brain tumors. Acta Neuropathol 116: 597–602 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

