The Mitochondrial DNA-Associated Protein SWIB5 Influences mtDNA Architecture and Homologous Recombination
- PMID: 28420746
- PMCID: PMC5466028
- DOI: 10.1105/tpc.16.00899
The Mitochondrial DNA-Associated Protein SWIB5 Influences mtDNA Architecture and Homologous Recombination
Abstract
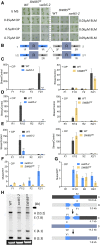
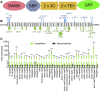
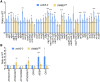
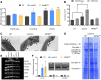
In addition to the nucleus, mitochondria and chloroplasts in plant cells also contain genomes. Efficient DNA repair pathways are crucial in these organelles to fix damage resulting from endogenous and exogenous factors. Plant organellar genomes are complex compared with their animal counterparts, and although several plant-specific mediators of organelle DNA repair have been reported, many regulators remain to be identified. Here, we show that a mitochondrial SWI/SNF (nucleosome remodeling) complex B protein, SWIB5, is capable of associating with mitochondrial DNA (mtDNA) in Arabidopsis thaliana Gain- and loss-of-function mutants provided evidence for a role of SWIB5 in influencing mtDNA architecture and homologous recombination at specific intermediate-sized repeats both under normal and genotoxic conditions. SWIB5 interacts with other mitochondrial SWIB proteins. Gene expression and mutant phenotypic analysis of SWIB5 and SWIB family members suggests a link between organellar genome maintenance and cell proliferation. Taken together, our work presents a protein family that influences mtDNA architecture and homologous recombination in plants and suggests a link between organelle functioning and plant development.
© 2017 American Society of Plant Biologists. All rights reserved.
Figures



Similar articles
-
A RAD52-like single-stranded DNA binding protein affects mitochondrial DNA repair by recombination.Plant J. 2012 Nov;72(3):423-35. doi: 10.1111/j.1365-313X.2012.05097.x. Epub 2012 Aug 30. Plant J. 2012. PMID: 22762281
-
Arabidopsis thaliana organellar DNA polymerase IB mutants exhibit reduced mtDNA levels with a decrease in mitochondrial area density.Physiol Plant. 2013 Sep;149(1):91-103. doi: 10.1111/ppl.12009. Epub 2012 Dec 13. Physiol Plant. 2013. PMID: 23167278
-
The plant-specific ssDNA binding protein OSB1 is involved in the stoichiometric transmission of mitochondrial DNA in Arabidopsis.Plant Cell. 2006 Dec;18(12):3548-63. doi: 10.1105/tpc.106.042028. Epub 2006 Dec 22. Plant Cell. 2006. PMID: 17189341 Free PMC article.
-
The plant mitochondrial genome: dynamics and maintenance.Biochimie. 2014 May;100:107-20. doi: 10.1016/j.biochi.2013.09.016. Epub 2013 Sep 26. Biochimie. 2014. PMID: 24075874 Review.
-
DNA Repair and the Stability of the Plant Mitochondrial Genome.Int J Mol Sci. 2020 Jan 3;21(1):328. doi: 10.3390/ijms21010328. Int J Mol Sci. 2020. PMID: 31947741 Free PMC article. Review.
Cited by
-
Phytohormones as Regulators of Mitochondrial Gene Expression in Arabidopsis thaliana.Int J Mol Sci. 2023 Nov 29;24(23):16924. doi: 10.3390/ijms242316924. Int J Mol Sci. 2023. PMID: 38069246 Free PMC article.
-
Disentangling the intertwined roles of mutation, selection and drift in the mitochondrial genome.Philos Trans R Soc Lond B Biol Sci. 2020 Jan 20;375(1790):20190173. doi: 10.1098/rstb.2019.0173. Epub 2019 Dec 2. Philos Trans R Soc Lond B Biol Sci. 2020. PMID: 31787045 Free PMC article. Review.
-
Transcriptome profiling of laser-captured crown root primordia reveals new pathways activated during early stages of crown root formation in rice.PLoS One. 2020 Nov 19;15(11):e0238736. doi: 10.1371/journal.pone.0238736. eCollection 2020. PLoS One. 2020. PMID: 33211715 Free PMC article.
-
GSyellow, a Multifaceted Tag for Functional Protein Analysis in Monocot and Dicot Plants.Plant Physiol. 2018 Jun;177(2):447-464. doi: 10.1104/pp.18.00175. Epub 2018 Apr 20. Plant Physiol. 2018. PMID: 29678859 Free PMC article.
-
Genome communication in plants mediated by organelle-n-ucleus-located proteins.Philos Trans R Soc Lond B Biol Sci. 2020 Jun 22;375(1801):20190397. doi: 10.1098/rstb.2019.0397. Epub 2020 May 4. Philos Trans R Soc Lond B Biol Sci. 2020. PMID: 32362260 Free PMC article. Review.
References
-
- Albinsky D., Masson J.E., Bogucki A., Afsar K., Vass I., Nagy F., Paszkowski J. (1999). Plant responses to genotoxic stress are linked to an ABA/salinity signaling pathway. Plant J. 17: 73–82.
-
- Andriankaja M., Dhondt S., De Bodt S., Vanhaeren H., Coppens F., De Milde L., Mühlenbock P., Skirycz A., Gonzalez N., Beemster G.T.S., Inzé D. (2012). Exit from proliferation during leaf development in Arabidopsis thaliana: a not-so-gradual process. Dev. Cell 22: 64–78. - PubMed
-
- Backert S., Nielsen B.L., Börner T. (1997). The mystery of the rings: structure and replication of mitochondrial genomes from higher plants. Trends Plant Sci. 2: 477–483.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

