Synergistic Antimicrobial Effects of Silver/Transition-metal Combinatorial Treatments
- PMID: 28420878
- PMCID: PMC5429853
- DOI: 10.1038/s41598-017-01017-7
Synergistic Antimicrobial Effects of Silver/Transition-metal Combinatorial Treatments
Abstract
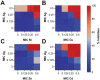
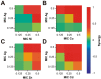
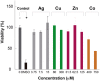
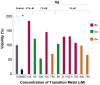
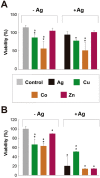
Due to the emergence of multi-drug resistant strains, development of novel antibiotics has become a critical issue. One promising approach is the use of transition metals, since they exhibit rapid and significant toxicity, at low concentrations, in prokaryotic cells. Nevertheless, one main drawback of transition metals is their toxicity in eukaryotic cells. Here, we show that the barriers to use them as therapeutic agents could be mitigated by combining them with silver. We demonstrate that synergism of combinatorial treatments (Silver/transition metals, including Zn, Co, Cd, Ni, and Cu) increases up to 8-fold their antimicrobial effect, when compared to their individual effects, against E. coli and B. subtilis. We find that most combinatorial treatments exhibit synergistic antimicrobial effects at low/non-toxic concentrations to human keratinocyte cells, blast and melanoma rat cell lines. Moreover, we show that silver/(Cu, Ni, and Zn) increase prokaryotic cell permeability at sub-inhibitory concentrations, demonstrating this to be a possible mechanism of the synergistic behavior. Together, these results suggest that these combinatorial treatments will play an important role in the future development of antimicrobial agents and treatments against infections. In specific, the cytotoxicity experiments show that the combinations have great potential in the treatment of topical infections.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

