Motion-Induced Position Shifts Activate Early Visual Cortex
- PMID: 28420952
- PMCID: PMC5376622
- DOI: 10.3389/fnins.2017.00168
Motion-Induced Position Shifts Activate Early Visual Cortex
Abstract
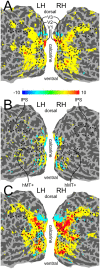
The ability to correctly determine the position of objects in space is a fundamental task of the visual system. The perceived position of briefly presented static objects can be influenced by nearby moving contours, as demonstrated by various illusions collectively known as motion-induced position shifts. Here we use a stimulus that produces a particularly strong effect of motion on perceived position. We test whether several regions-of-interest (ROIs), at different stages of visual processing, encode the perceived rather than retinotopically veridical position. Specifically, we collect functional MRI data while participants experience motion-induced position shifts and use a multivariate pattern analysis approach to compare the activation patterns evoked by illusory position shifts with those evoked by matched physical shifts. We find that the illusory perceived position is represented at the earliest stages of the visual processing stream, including primary visual cortex. Surprisingly, we found no evidence of percept-based encoding of position in visual areas beyond area V3. This result suggests that while it is likely that higher-level visual areas are involved in position encoding, early visual cortex also plays an important role.
Keywords: functional MRI; motion-induced position shifts; position perception; spatial vision; striate and extrastriate cortex.
Figures


References
LinkOut - more resources
Full Text Sources
Other Literature Sources

